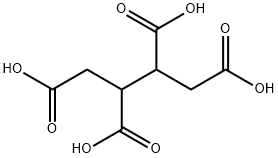
1,2,3,4-Butanetetracarboxylic acid
- CAS No.
- 1703-58-8
- Chemical Name:
- 1,2,3,4-Butanetetracarboxylic acid
- Synonyms
- BTCA;1,2,3,4-ButaneTetraCarboxylic;1,2,3,4-Butanetetrac;Butanetetraacetic acid;butanetetracarboxylicacid;1,2,3,4-TETRACARBOXYBUTANE;1,2,3,4-ButanetetracarboxyL;1,2,3,4-BUTANETETRACARBOXYLIC ACID;2,3-Bis(carboxymethyl)succinic acid;butane-1,2,3,4-tetracarboxylic acid
- CBNumber:
- CB5251171
- Molecular Formula:
- C8H10O8
- Molecular Weight:
- 234.16
- MDL Number:
- MFCD00002722
- MOL File:
- 1703-58-8.mol
- MSDS File:
- SDS
| Melting point | 193-197 °C |
|---|---|
| Boiling point | 296.47°C (rough estimate) |
| Density | 1.5040 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5800 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 118g/l |
| form | Crystalline Powder |
| pka | pK1: 3.43;pK2: 4.58;pK3: 5.85;pK4: 7.16 (25°C) |
| color | White |
| Water Solubility | >=10 g/100 mL at 19 ºC |
| BRN | 1729167 |
| InChIKey | GGAUUQHSCNMCAU-UHFFFAOYSA-N |
| LogP | -1.41 at 25℃ |
| CAS DataBase Reference | 1703-58-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 5A3KY454MZ |
| EPA Substance Registry System | 1,2,3,4-Butanetetracarboxylic acid (1703-58-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H319 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P312-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36-36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| RIDADR | 3261 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EK6100000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29171990 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1720 mg/kg | |||||||||
| NFPA 704 |
|
1,2,3,4-Butanetetracarboxylic acid price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.14924 | 1,2,3,4-Butanetetracarboxylic acid for synthesis | 1703-58-8 | 50g | $60 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14924 | 1,2,3,4-Butanetetracarboxylic acid for synthesis | 1703-58-8 | 250g | $118 | 2024-03-01 | Buy |
| Sigma-Aldrich | 257303 | 1,2,3,4-Butanetetracarboxylic acid 99% | 1703-58-8 | 100g | $119 | 2024-03-01 | Buy |
| Sigma-Aldrich | 257303 | 1,2,3,4-Butanetetracarboxylic acid 99% | 1703-58-8 | 500g | $257 | 2024-03-01 | Buy |
| Alfa Aesar | A16612 | 1,2,3,4-Butanetetracarboxylic acid, 98+% | 1703-58-8 | 100g | $64.7 | 2024-03-01 | Buy |
1,2,3,4-Butanetetracarboxylic acid Chemical Properties,Uses,Production
Chemical Properties
White leaf-shaped or needle-shaped crystals. melting point 236-237℃ for dextrorotatory-levorotatory type, 189℃ for endocyclic type. Solubility of the product in water (g/100g water): 30℃, 18.5; 50℃, 45.5; 70℃, 81.8. Soluble in ethanol. There is almost no hygroscopicity, and the purity does not decrease when left for more than two years.
Uses
1,2,3,4-Butanetetracarboxylic acid (BTCA) is a perfect formaldehyde-free durable press (DP) finishing agent. It has high activity of reaction and no irritant odor.It is used as cotton, hemp, silk and other cellulosic fibers, anti-wrinkle and anti-shrink agent, with the finishing agent for fabric finishing, can improve the shaping effect and the fabric does not contain formaldehyde.
BTCA, catalyzed by sodium hypophosphite (SHP), brings good antiwrinkle property to cotton fabrics and causes significant strength losses due to cross-linking and acidic degradation of cellulose simultaneously.
Uses
1,2,3,4-Butanetetracarboxylic acid is used as a cross linking agent to prepare cotton cellulose in order to improve anti-prilling and flame retardant properties. It is used as a spacer in the cross-linking of titania nanoparticles to cotton. Further, it is used as a nonformaldehyde durable press finishing agent.
Preparation
1,2,3,4-butanetetracarboxylic acid is obtained by oxidation of tetrahydrophthalic anhydride.
1,2,3,4-butanetetracarboxylic acid is prepared by oxidative cleavage of tetraphthalic acid or anhydride by oxidation with ozone-containing gas, followed by oxygen-containing gas, with the mixture then being heated with a peroxide, e.g. H2 O2, at 100° C. to produce the butanetetracarboxylic acid.
Process for preparing 1,2,3,4-butanetetracarboxylic acid
General Description
Leaflets (from water) or white powder.
Air & Water Reactions
Soluble in water.
Reactivity Profile
1,2,3,4-Butanetetracarboxylic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 1,2,3,4-Butanetetracarboxylic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1,2,3,4-Butanetetracarboxylic acid emits acrid smoke and irritating fumes.
Fire Hazard
Flash point data for 1,2,3,4-Butanetetracarboxylic acid are not available; however, 1,2,3,4-Butanetetracarboxylic acid is probably combustible.
Flammability and Explosibility
Not classified
1,2,3,4-Butanetetracarboxylic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1179 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18648 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21639 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29885 | 58 |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | stella@zetchem.com | China | 2136 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2964 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8817 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2993 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
View Lastest Price from 1,2,3,4-Butanetetracarboxylic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-02 | 1,2,3,4-Butanetetracarboxylic acid/BTCA
1703-58-8
|
US $0.00 / Kg/Drum | 1KG | 98%min | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-09-12 | 1,2,3,4-Butanetetracarboxylic acid
1703-58-8
|
US $0.00 / kg | 1kg | 99% | 50000KG/month | Hebei Mojin Biotechnology Co.,Ltd | |
 |
2023-10-11 | 1,2,3,4-Butanetetracarboxylic acid
1703-58-8
|
US $100.00 / bag | 1bag | 99 | 5000 | Hebei Fengqiang Trading Co., LTD |
-

- 1,2,3,4-Butanetetracarboxylic acid/BTCA
1703-58-8
- US $0.00 / Kg/Drum
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- 1,2,3,4-Butanetetracarboxylic acid
1703-58-8
- US $0.00 / kg
- 99%
- Hebei Mojin Biotechnology Co.,Ltd
-

- 1,2,3,4-Butanetetracarboxylic acid
1703-58-8
- US $100.00 / bag
- 99
- Hebei Fengqiang Trading Co., LTD
1703-58-8(1,2,3,4-Butanetetracarboxylic acid)Related Search:
1of4