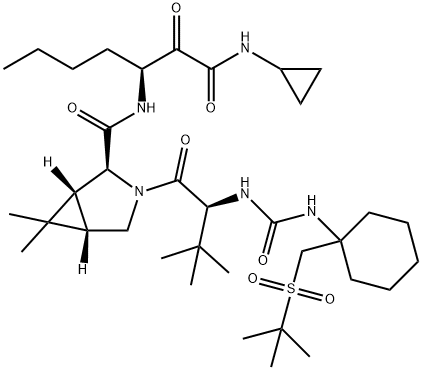
Narlaprevir
- CAS No.
- 865466-24-6
- Chemical Name:
- Narlaprevir
- Synonyms
- Sch 900518;Narlaprevir;Sch 900518 Narlaprevir;Narlaprevir(SCH 900518 );SCH 900518;SCH900518;SCH-900518;(1R,2S,5S)-N-[(1S)-1-[(Cyclopropylamino)oxoacetyl]pentyl]-3-[(2S)-2-[[[[1-[[(1,1-dimethylethyl)sulfonyl]methyl]cyclohexyl]amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide;3-Azabicyclo[3.1.0]hexane-2-carboxamide, N-[(1S)-1-[2-(cyclopropylamino)-2-oxoacetyl]pentyl]-3-[(2S)-2-[[[[1-[[(1,1-dimethylethyl)sulfonyl]methyl]cyclohexyl]amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-, (1R,2S,5S)-
- CBNumber:
- CB52554290
- Molecular Formula:
- C36H61N5O7S
- Molecular Weight:
- 707.96
- MDL Number:
- MFCD16038932
- MOL File:
- 865466-24-6.mol
- MSDS File:
- SDS
| Melting point | 152 - 155°C |
|---|---|
| Density | 1.21 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.84±0.20(Predicted) |
| color | White to Off-White |
| Stability | Hygroscopic |
| FDA UNII | 2857LA2O07 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Narlaprevir price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | N379115 | Narlaprevir | 865466-24-6 | 2.5mg | $305 | 2021-12-16 | Buy |
| Biorbyt Ltd | orb611501 | Narlaprevir | 865466-24-6 | 1g | $615.4 | 2021-12-16 | Buy |
| ChemScene | CS-0445 | Narlaprevir 95.06% | 865466-24-6 | 10mg | $670 | 2021-12-16 | Buy |
| ApexBio Technology | A3643 | Narlaprevir | 865466-24-6 | 10mg | $817 | 2021-12-16 | Buy |
| ChemScene | CS-0445 | Narlaprevir 95.06% | 865466-24-6 | 50mg | $1085 | 2021-12-16 | Buy |
Narlaprevir Chemical Properties,Uses,Production
Description
Narlaprevir was approved as a treatment for genotype 1 HCV and serves as a class 2 HCV NS3 serine protease inhibitor. In clinical trials, it showed a rapid and steady decline in HCV-RNA levels in both previously treated and treatment-naive patients when used in combination with ritonavir and PEG-IFN-α. This combination ultimately led to ≥50% of patients with undetectable HCV-RNA levels after a second period of treatment. Narlaprevir also has demonstrated activity against HCV mutations resistant to other treatments such as boceprevir and telaprevir. The unique activity of this drug can be attributed to a critical electrophilic α-keto-amide “warhead”, which covalently reacts with an HCV NS3 protease active-site serine residue involved in the HCV viral replication process. Because of their essential roles in viral replication, HCV NS3 and NS5B proteases have recently become key targets for HCV drug development. Strategically, the development of narlaprevir stems specifically from the pursuit of a single-diastereomer, second generation HCV protease inhibitor, which would provide in vitro potency and pharmacokinetic profile improvements over the structurally related antiviral drug boceprevir,which exists as a mixture of diastereomers. After the R-Pharm pharmaceutical group obtained the license to manufacture narlaprevir from Merck in 2012, further development of the drug was realized through collaborations with Schering-Plough and Texas Liver Institute.
Uses
Narlaprevir is an NS3/4A protease inhibitor used in the treatment of hepatitis C virus, HCV.
Definition
ChEBI: Narlaprevir is an azabicyclohexane that is (1R,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane substituted by [(3S)-1-(cyclopropylamino)-1,2-dioxoheptan-3-yl]aminoacyl and N-({1-[(tert-butylsulfonyl)methyl]cyclohexyl}carbamoyl)-3-methyl-L-valyl groups at positions 2S and 3, respectively. It is a hepatitis C virus (HCV) NS3/4A serine protease inhibitor (Ki = 6 nM) that is used for the treatment of chronic hepatitis C. It has a role as a hepatitis C protease inhibitor, an antiviral drug, an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor and an anticoronaviral agent. It is a sulfone, a member of ureas, a tertiary carboxamide, an azabicyclohexane, a pyrrolidinecarboxamide, a secondary carboxamide and a member of cyclopropanes.
Synthesis
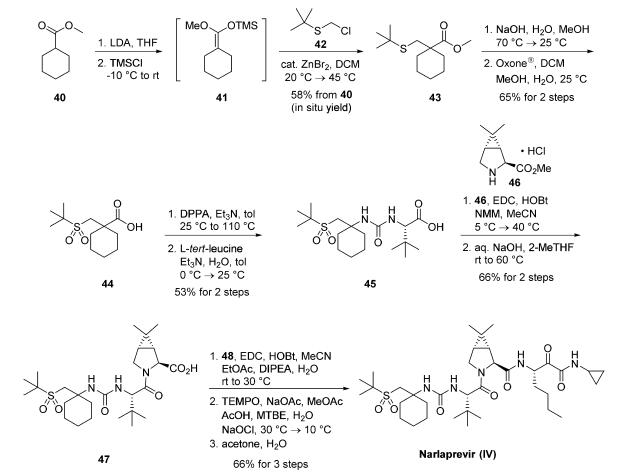
A kilogram-scale synthetic route to narlaprevir has been
reported and proceeds strategically through the union of urea
45, bicyclic amine intermediate 46, and amine salt 48 . Preparation of urea 45 begins with commercial
cyclohexanecarboxylic acid methyl ester (40), which was
treated with freshly prepared LDA and TMSCl in THF to
provide silyl enol ether 41. This intermediate was
immediately reacted with commercial 2-[(chloromethyl)thio]-
2-methylpropane (42) under Lewis acid conditions (ZnBr2) to
provide ester 43 in 58% yield over the two-step process.17,19 A
solution of crude 43 was subjected to saponification conditions
(NaOH, H2O, MeOH) and sulfide oxidation with oxone in
DCM/MeOH, leading to the target sulfone 44 in 65% yield.
From 44, a Curtius rearrangement delivered an isocyanate
intermediate that could be trapped with L-tert-leucine, forming
the desired urea 45 in 53% over the two-step sequence.17,19
Coupling 45 with commercially available bicyclic amine 46
under peptide coupling conditions (EDC, HOBt, NMM) led to
the desired amide in 79% yield, which was then saponified with
aqueous NaOH in 2-methyltetrahydrofuran (2-MeTHF) to
provide acid intermediate 47 (84% yield). This intermediate
was coupled with amine salt 48 with EDC and HOBt, providing the penultimate
intermediate to narlaprevir. Completion of the synthesis relied
upon installation of the essential |á-keto-amide functionality,
which was accomplished by |á-hydroxy amide oxidation using
TEMPO-catalyzed conditions. A final recrystallization from
acetone/water completed synthesis of narlaprevir (IV) in 83%
yield. It is worth noting that this overall route was used to generate >1 kg of narlaprevir and required no chromatographic
separation steps.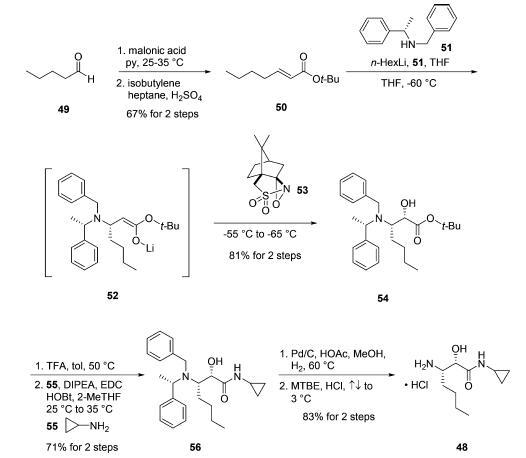
Amine salt 48 was prepared by first subjecting commercially
available pentanal (49) to Knoevenagel condensation conditions
using malonic acid followed by conversion of the
resulting acid to the corresponding t-butyl ester 50 by reaction
with H2SO4 and isobutylene. The key
transformation for establishing the requisite stereocenter in
intermediate 48 relied on an asymmetric conjugate addition of
a bis-protected lithiated amine followed by enolate trap with an
electrophilic source of oxygen. In practice, treatment of |á-
methyl-N-(phenylmethyl)-(|áS)-benzenemethanamine (51)
with n-hexyllithium resulted in stereoselective 1,4-addition to
enone 50. Subjection of lithium enolate intermediate 52 to
(1S)-(+)-(10-camphorsulfonyl)oxaziridine (53) then furnished
the |á-hydroxyl group and delivered the syn-amino alcohol
derivative 54 in 81% yield for the two-step protocol. tert-Butyl
ester removal was realized by exposure of 54 to TFA in warm
toluene. Subsequent coupling of the resulting acid with
cyclopropylamine (55) utilizing EDC and HOBt conditions
provided cyclopropyl amide 56 in 71% yield from 54. Finally,
hydrogenolytic removal of the benzyl groups from the |?-amine
followed by subjection of the product to refluxing HCl
provided amine salt 48 in 83% yield.19a
target
NS3 protease
References
[1]. arasappan a, bennett f, bogen s l, et al. discovery of narlaprevir (sch 900518): a potent, second generation hcv ns3 serine protease inhibitor. acs medicinal chemistry letters, 2010, 1(2): 64-69.
[2]. tong x, arasappan a, bennett f, et al. preclinical characterization of the antiviral activity of sch 900518 (narlaprevir), a novel mechanism-based inhibitor of hepatitis c virus ns3 protease. antimicrobial agents and chemotherapy, 2010, 54(6): 2365-2370.
[3]. wang h, geng l, chen b z, et al. computational study on the molecular mechanisms of drug resistance of narlaprevir due to v36m, r155k, v36m+ r155k, t54a, and a156t mutations of hcv ns3/4a protease. biochemistry and cell biology, 2014, 92(5): 357-369.
Narlaprevir Preparation Products And Raw materials
Raw materials
Preparation Products
Narlaprevir Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Shanghai Safer Pharmaceutical Technology Co., Ltd. | 021-31761238 | sales@saferph.com | CHINA | 897 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32161 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30239 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9312 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25356 | 58 |
| Nanjing Fred Technology Co., Ltd | +86-25-84696168 +86-15380713688 | Austin@fredbio.com | China | 2428 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
View Lastest Price from Narlaprevir manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-13 | Narlaprevir
865466-24-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-10 | Narlaprevir
865466-24-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Narlaprevir
865466-24-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Narlaprevir
865466-24-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd