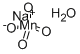ChemicalBook >> CAS DataBase List >>SODIUM PERMANGANATE MONOHYDRATE
SODIUM PERMANGANATE MONOHYDRATE
- CAS No.
- 79048-36-5
- Chemical Name:
- SODIUM PERMANGANATE MONOHYDRATE
- Synonyms
- Sodium permanganate hydrate;SODIUM PERMANGANATE-1-HYDRATE;Sodium permangate monohydrate;SODIUM PERMANGANATE MONOHYDRATE;Sodium permanganate hydrate, 97+%;SODIUM PERMANGANATE MONOHYDRATE, 97+%;SODIUM PERMANGANATE MONOHYDRATE ISO 9001:2015 REACH
- CBNumber:
- CB5270367
- Molecular Formula:
- H2MnNaO5
- Molecular Weight:
- 159.94
- MDL Number:
- MFCD00149165
- MOL File:
- 79048-36-5.mol
- MSDS File:
- SDS
Last updated:2025-01-27 09:38:02
| solubility | Acetonitrile (Slightly), Methanol (Slightly), Water (Slightly) |
|---|---|
| form | Solid |
| color | Dark Purple to Black |
| FDA UNII | Q90Z1KJ1HK |
| UNSPSC Code | 12352302 |
| NACRES | NA.21 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H272-H314 |
| Precautionary statements | P210-P260-P280-P303+P361+P353-P305+P351+P338+P310 |
| Hazard Codes | O,C |
| Risk Statements | 8-34 |
| Safety Statements | 17-26-36/37/39-45 |
| RIDADR | UN 1503 5.1/PG 2 |
| WGK Germany | 3 |
| F | 3-10 |
SODIUM PERMANGANATE MONOHYDRATE Chemical Properties,Uses,Production
Chemical Properties
Sodium permanganate monohydrate is decomposed in alkali, similar to potassium permanganate. Easy deliquescence, soluble in water. It is flammable and releases iodine vapor. Soluble in water, ethanol, ether and liquid ammonia.
Uses
Sodium permanganate monohydrate can be used as an oxidant, bactericide, antidote, and a substitute for potassium permanganate.
reaction suitability
reagent type: oxidant
SODIUM PERMANGANATE MONOHYDRATE Preparation Products And Raw materials
Raw materials
Preparation Products
79048-36-5(SODIUM PERMANGANATE MONOHYDRATE)Related Search:
SODIUM PERMANGANATE MONOHYDRATE
SODIUM PERMANGANATE MONOHYDRATE, 97+%
SODIUM PERMANGANATE-1-HYDRATE
Sodium permanganate hydrate, 97+%
Sodium permanganate hydrate
Sodium permangate monohydrate
SODIUM PERMANGANATE MONOHYDRATE ISO 9001:2015 REACH
79048-36-5
NaMnO4H2O
MnO4NaH2O
H2OMnO4Na
Synthetic Reagents
Others
Oxidation
Others
Oxidation
Synthetic Reagents