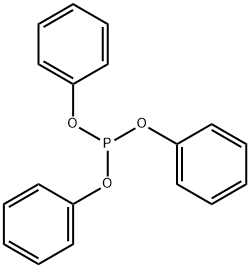
Triphenyl phosphite
- CAS No.
- 101-02-0
- Chemical Name:
- Triphenyl phosphite
- Synonyms
- TPO;P(OPh)3;(PhO)3P;Weston TPP;PHENYL PHOSPHITE;p36;EFED;P 36;jp360;westontpp
- CBNumber:
- CB5296820
- Molecular Formula:
- C18H15O3P
- Molecular Weight:
- 310.28
- MDL Number:
- MFCD00003032
- MOL File:
- 101-02-0.mol
- MSDS File:
- SDS
| Melting point | 22-24 °C (lit.) |
|---|---|
| Boiling point | 360 °C (lit.) |
| Density | 1.184 g/mL at 25 °C (lit.) |
| vapor density | 10.7 (vs air) |
| vapor pressure | 5 mm Hg ( 205 °C) |
| refractive index |
n |
| Flash point | 425 °F |
| storage temp. | Store below +30°C. |
| solubility | methanol: 25 mg/mL, clear |
| form | Liquid |
| color | Clear colorless to pale yellow |
| PH | 1 (200g/l, H2O, 20℃) |
| Water Solubility | insoluble |
| Sensitive | Air & Moisture Sensitive |
| BRN | 1079456 |
| Dielectric constant | 3.2(24℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | HVLLSGMXQDNUAL-UHFFFAOYSA-N |
| CAS DataBase Reference | 101-02-0(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | TRIPHENYL PHOSPHITE |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | TPO |
| FDA UNII | 9P45GRD24X |
| NIST Chemistry Reference | Phosphorous acid, triphenyl ester(101-02-0) |
| EPA Substance Registry System | Triphenyl phosphite (101-02-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H317-H319-H410 | |||||||||
| Precautionary statements | P261-P273-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,N,Xi | |||||||||
| Risk Statements | 36/38-50/53 | |||||||||
| Safety Statements | 28-60-61-28A-26 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | TH1575000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29209085 | |||||||||
| Hazardous Substances Data | 101-02-0(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in rat: 444mg/kg | |||||||||
| NFPA 704 |
|
Triphenyl phosphite price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T84654 | Triphenyl phosphite 97% | 101-02-0 | 5g | $30.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | T84654 | Triphenyl phosphite 97% | 101-02-0 | 3kg | $267 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00551 | Triphenyl phosphite for synthesis | 101-02-0 | 100mL | $45.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00551 | Triphenyl phosphite for synthesis | 101-02-0 | 1L | $97.1 | 2024-03-01 | Buy |
| TCI Chemical | T0510 | Triphenyl Phosphite >97.0%(GC) | 101-02-0 | 25g | $25 | 2024-03-01 | Buy |
Triphenyl phosphite Chemical Properties,Uses,Production
Secondary antioxidants
Triphenyl phosphite is called for short TPP, it is also known as triphenoxytitanium phosphine, it is phosphite-based compound, it is a secondary antioxidant, it has light stabilizing effect, it is applicable to polyvinyl chloride, polypropylene, polystyrene, poly acetate, ABS resin, epoxy resin and the like. Triphenyl phosphite is widely used as polyvinyl chloride chelating agent, when the metal soap is used as stabilizer, coordinating with this product can reduce the harm of the metal chlorides, it can keep transparency of product and inhibit the change in color. Further triphenyl phosphate is also used as flame retardant plasticizer.
Phosphite phenyl diisooctyl can be obtained by the transesterification reaction of triphenyl phosphite and isooctanol in the presence of catalyst, phosphite phenyl diisooctyl can be used as secondary antioxidant. It has good resistance to discoloration, it can increase oxidation resistance and light stability. It has chelating action in PVC, low toxicity, it can be used in plastic medical devices.
In addition, this product in the presence of sodium methoxide can proceed the transesterification reaction with methanol to form trimethyl phosphite.
Triphenyl phosphite is as raw material, it can react with octanol in the solution of sodium methoxide, octyl diphenyl phosphite can be prepared.
The above information are collated by Xiaonan edit Chemicalbook (2016-12-03).
Physical and chemical properties
It is colorless or pale yellow oily liquid. It has slightly with phenol odor. Molecular weight is 310. Color (APHA) <50. Acid value is <0.5mgKOH/g. Phosphorus content is 10%. The relative density is 1. 180~1.186 (25°/ 15.5℃). Viscosity is 12 mPa•s (38℃), 4.5mPa•s (99℃). The freezing point is 19~24℃. Melting point is 22~25℃. Boiling point is 220℃ (1333Pa). Flash point (open cup) is 218.3℃, ignition point is 243℃. Refractive index is 1.5880 ~1.5900 (25℃). Solubility (g/100g solvent): methanol> 10, benzene> 10 acetone> 10, it is insoluble in water. Triphenyl phosphate has irritating to the skin, rat oral LD50 is 2800mg/kg body weight, it is used as polymer antioxidant and stabilizer, it has better synergy effect with many phenolic antioxidants. By effect of phosphorus trichloride and phenol can get triphenyl phosphite.
Chemical properties
It is colorless to pale yellow monoclinic crystal below room temperature. It is colorless light yellow transparent oily liquid at room temperature or higher, it has pungent odor. It is insoluble in water, soluble in organic solvents such as ethanol, ethyl ether, acetone, benzene and the like.
Uses
(1) Triphenyl phosphite is used as chelating agents, plastics antioxidant, alkyd resin and pesticide intermediate raw material.
(2) It is used for synthetic rubber and resin stabilizer, PVC antioxidant, alkyd resin and pesticide intermediate raw material.
(3) Triphenyl phosphite is used as secondary antioxidants, it has effect of light stability, it is suitable for polyvinyl chloride, polypropylene, polystyrene, polyester, ABS resin, and epoxy resin. (4) It is used as chelating agent in PVC products, it can enable products to maintain transparency, and suppresse color change. It can also be used for the production of trimethyl phosphite.
(5) Antioxidants, stabilizers TPPi is mainly applied to polyvinyl chloride, polyethylene, polypropylene, polystyrene, polyester, ABS resin, epoxy resin, synthetic rubber antioxidant stabilizer for PVC vinyl products as chelating agent, when the metal is as based stabilizer, coordinating with the product can reduce the harm of metal chloride
(6) Chelating agent. Plastic products antioxidant. The synthesis of alkyd resins and polyester resins.
(7) Chelating agent. Plastic products antioxidant. The synthesis of alkyd resins and polyester resins.
(8) Chelating agent, it is widely used in all kinds of PVC products, it can enable products to maintain its transparency and suppress color change, and it can increase the antioxidant and light and heat stability of the primary stabilizer. In addition, the product is also used for PE, PP, ABS, SBS and other products, and it can be used for pesticide intermediates. It can be used for synthetic alkyd resins and polyester materials and polyester resins.
Uses
Triphenyl phosphite (TPP) is the chemical compound with the formula P(OC6H5)3. This colourless viscous liquid is the ester of phosphorous acid and phenol. It is used as a ligand in organometallic chemistry. Nickel complexes of this ligand are homogeneous catalysts for the hydrocyanation of alkenes.
Production methods
It can be obtained from phenol and phosphorus trichloride. Raw material consumption (kg/t) phenol (freezing point ≥40.4 ℃) 940 phosphorus trichloride (99%) 480
Toxic Effects
The primary toxic effects of triphenyl phosphite are exerted on the nervous system of susceptible animals. The univeral signs of triphenyl phosphite neurotoxicity result from the irreversible inhibition of acetylcholinesterase (AChE) at cholinergic synapses in the central, peripheral and autonomic nervous system. Triphenyl phosphite is also capable of producing characteristic delayed neurotoxic effects that are manifested several days or weeks after even minimal drug exposure. Such actions are not related to AChE inhibition and the precise biochemical mechanism(s) leading to the delayed neurotoxicity symptoms are largely unresolved.
Chemical Properties
Water-white to pale-yellow solid or oily liquid; pleasant odor. Combustible.
Uses
Stabilizer/antioxidant for vinyl plastics and polyethylene, polypropylene, styrene copolymers, and rubber.
Uses
Chemical intermediate, stabilizer systems for resins, metal scavenger, diluent for epoxy resins.
Uses
Triphenyl phosphite can be used:
- As a source of phosphorus and as a ligand for the synthesis of transition metal phosphide nanoparticles via heating-up process.
- To convert alcohols to alkyl halides.
- As a peptide coupling agent.
- As a low-temperature source of singlet oxygen after forming an adduct with ozone.
- To synthesize bromotriphenoxyphosphonium bromide, a brominating agent, by reacting with bromine.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Organophosphates, such as Triphenyl phosphite, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
Triphenyl phosphite (TPP) is a skin irritant and sensitizer in humans and is neurotoxic in laboratory animals.Systemic effects have not been reported in humans.
reaction suitability
Buchwald-Hartwig Cross-Coupling Reaction; Heck Reaction; Hiyama Coupling; Negishi Couplingp; Sonogashira Coupling; Stille Coupling; Suzuki-Miyaura Coupling.
Safety Profile
Poison by intraperitoneal and subcutaneous routes. Moderately toxic by ingestion. An experimental eye and severe human skin irritant. Combustible when exposed to heat or flame. To fight fire, use CO2, mist, dry chemical. When heated to decomposition it emits toxic fumes of POx. See also PHENOL.
Synthesis
1120.5 g (11.90 mol) of phenol (3.3 eq.) are placed in a 3-necked, 3-liter flask filled with nitrogen. 494.0 g (3.60 mol) of phosphorus trichloride is charged into a dropping funnel, and 864.5 g of distilled water is poured into an absorption flask for trapping hydrogen chloride. The reaction mixture is heated in an oil bath to a temperature of 45 ° C with stirring, and phosphorus trichloride is added dropwise over 240 minutes. Next, the reaction mixture is stirred for 20 minutes, heated to a temperature of 70 ° C for 10 minutes, kept at this temperature for 10 minutes, heated to a temperature of 160 ° C for 30 minutes and kept for 1.5 hours. Disconnect and weigh the absorption flask with an aqueous solution of hydrogen chloride and get 1242.0 g of hydrochloric acid (30.4 wt%). Replace the reflux condenser with a distillation nozzle with a once-through cooler and a receiving flask. The reaction flask is heated to a temperature of 170 ° C and maintained for 60 minutes under vacuum at a residual pressure of 300-350 mm RT. Art. Gradually reduce the vacuum to a residual pressure of 10 mm RT. Art. and phenol distillate 149.3 g is collected. After phenol is distilled off, triphenyl phosphite weighing 1091.3 g is obtained.
Purification Methods
Its ethereal solution is washed succesively with aqueous 5% NaOH, distilled water and saturated aqueous NaCl, then dried with Na2SO4 and distilled under vacuum after evaporating the diethyl ether. [Walsh J Am Chem Soc 81 3023 1959, Verkade & Coskren in Organo Phosphorus Compounds (Kosolapoff & Maier eds) Wiley Vol 6 pp 211-577 1973, Beilstein 6 IV 695.]
Triphenyl phosphite Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | sales@tjzxchem.com | China | 563 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20284 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 | hjt@hong-jin.com | China | 228 | 58 |
Related articles
- Triphenyl Phosphite: A Comprehensive Overview
- Triphenyl phosphite (TPhP) is a versatile chemical compound that serves as a critical element in various industrial and chemic....
- May 14,2024
- Triphenyl phosphite: synthesis, applications in organic synthesis and safety
- Triphenyl phosphite has versatile applications in organic synthesis as a reactant and catalyst but has potential safety hazard....
- Dec 19,2023
- Triphenyl phosphite: properties and applications
- Triphenyl phosphite plays a crucial role in organic synthesis, polymer preparation, and metal coordination chemistry, offering....
- Jul 12,2023
View Lastest Price from Triphenyl phosphite manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-30 | Triphenyl phosphite
101-02-0
|
US $10.00 / kg | 1kg | 99.5% | 100 TON | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-11 | Triphenyl Phosphite
101-02-0
|
US $5.00-2.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-20 | JADEWIN TPP
101-02-0
|
US $0.00 / KG | 25KG | 98% | 20 tons | QINGDAO JADE NEW MATERIAL TECNOLOGY CO.,LTD |
-

- Triphenyl phosphite
101-02-0
- US $10.00 / kg
- 99.5%
- Hebei Weibang Biotechnology Co., Ltd
-

- Triphenyl Phosphite
101-02-0
- US $5.00-2.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- JADEWIN TPP
101-02-0
- US $0.00 / KG
- 98%
- QINGDAO JADE NEW MATERIAL TECNOLOGY CO.,LTD