Phosphorus trichloride
- CAS No.
- 7719-12-2
- Chemical Name:
- Phosphorus trichloride
- Synonyms
- PCl3;Phosphorous trichloride;PHOSPHORUS CHLORIDE;trichlorophosphane;phosphore(trichlorurede);Trichlorophosphine;Phosphorous chloride;PICl;2125683;Fosfortrichloride
- CBNumber:
- CB6459046
- Molecular Formula:
- Cl3P
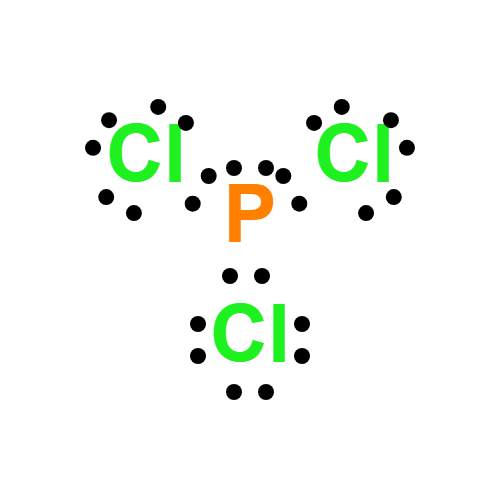
Lewis structure
- Molecular Weight:
- 137.33
- MDL Number:
- MFCD00011438
- MOL File:
- 7719-12-2.mol
- MSDS File:
- SDS
| Melting point | -112 °C |
|---|---|
| Boiling point | 74-78 °C(lit.) |
| Density | 1.574 g/mL at 25 °C |
| vapor density | 4.75 (vs air) |
| vapor pressure | 23.32 psi ( 55 °C) |
| refractive index |
n |
| Flash point | 76°C |
| storage temp. | Store at RT. |
| solubility | Soluble in benzene, carbon sulfide, ether, chloroform, carbon tetrachloride. |
| form | Liquid |
| Specific Gravity | approximate 1.6 |
| color | Yellow |
| Odor | Pungent, irritating odor |
| PH Range | 1 |
| PH | 1 (20°C, 5g/L in H2O) |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Merck | 14,7358 |
| BRN | 969177 |
| Exposure limits | TLV-TWA 1.12 mg/m3 (0.2 ppm) (ACGIH), 2.8 mg/m3 (0.5 ppm) (OSHA). |
| Dielectric constant | 3.7(18℃) |
| Stability | Stable, but light sensitive. Incompatible with water, many metals, fluorine, acids, variety of organic materials including acids, alcohols and reducing agents. Reaction with water is violent and yields toxic gas. |
| CAS DataBase Reference | 7719-12-2(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | PHOSPHOROUS TRICHLORIDE |
| EWG's Food Scores | 2 |
| FDA UNII | M97C0A6S8U |
| NIST Chemistry Reference | Phosphorus trichloride(7719-12-2) |
| EPA Substance Registry System | Phosphorus trichloride (7719-12-2) |
| UNSPSC Code | 12352302 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H330-H314-H373 | |||||||||
| Precautionary statements | P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P314 | |||||||||
| Hazard Codes | T+,C | |||||||||
| Risk Statements | 14-26/28-29-35-48/20-40-37 | |||||||||
| Safety Statements | 26-36/37/39-45-7/8-43-28 | |||||||||
| RIDADR | UN 3390 6.1/PG 1 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 0.2 ppm (1.5 mg/m3), STEL: 0.5 ppm (3 mg/m3) | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | TH3675000 | |||||||||
| F | 21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28121043 | |||||||||
| Hazardous Substances Data | 7719-12-2(Hazardous Substances Data) | |||||||||
| IDLA | 25 ppm | |||||||||
| NFPA 704 |
|
Phosphorus trichloride price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 157791 | Phosphorus trichloride 99% | 7719-12-2 | 50g | $59.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 157791 | Phosphorus trichloride 99% | 7719-12-2 | 1kg | $165 | 2024-03-01 | Buy |
| Alfa Aesar | 022988 | Phosphorus(III) chloride, 99.997% (metals basis) | 7719-12-2 | 25g | $288 | 2023-06-20 | Buy |
| Alfa Aesar | 022988 | Phosphorus(III) chloride, 99.997% (metals basis) | 7719-12-2 | 100g | $762 | 2023-06-20 | Buy |
| Strem Chemicals | 93-1588 | Phosphorus(III) chloride (99.998%-P) PURATREM | 7719-12-2 | 10g | $90 | 2024-03-01 | Buy |
Phosphorus trichloride Chemical Properties,Uses,Production
Chemical Properties
Phosphorus trichloride (phosphorus chloride, PCl3) is a colourless transparent motile liquid (the boiling point is 76 °C); it fumes in air. It mixes with diethyl ether, petrol, chloroform, carbon disulfide and dichloroethane in all ratios: It can be easily destroyed with water, acids and alcohols. Phosphorus trichloride vapours hydrolyse even in humid air. PCl3 is a strong oxidizer and will readily react with many organic compounds.
Phosphorus trichloride is very poisonous; it causes serious skin burns and can cause death when enters the human body (even in small quantities). The maximum allowable concentration of phosphorus trichloride Vapours in industry is 0.5 mg/m3.
Uses
Phosphorus trichloride is used to prepare phosphine and other phosphorus compounds; used during electrodeposition of metal on rubber and for making pesticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, germicides, medicinal products, and other chemicals.
Preparation
Phosphorus trichloride is prepared by reacting white phosphorus with dry chlorine present in limited quantity. Excess chlorine will yield phosphorus pentachloride, PCl5.
P4 + 6Cl2 → 4PCl3
P4 + 10Cl2 → 4PCl5
The compound is prepared in a retort attached to inlet tubes for dry chlorine and dry carbon dioxide and a distillation flask. White phosphorus is placed on sand in the retort. All air, moisture, and any phosphorus oxide vapors present in the apparatus are expelled by passing dry carbon dioxide.
Dry chlorine is then introduced into the apparatus. If a flame appears on phosphorus it indicates presence of excess chlorine. In that event, the rate of chlorine introduction should be decreased. For obtaining phosphorus trichloride, flame should appear at the end of the chlorine-entry tube. The trichloride formed is collected by condensation in the distillation flask. A soda lime tube is attached to the apparatus to prevent moisture entering the flask.
Phosphorus trichloride also can be prepared by reducing phosphorus oxychloride vapors with carbon at red heat:
POCl3 + C → PCl3 + CO
Description
Phosphorus trichloride, PCl3, is a clear, colorless, fuming, corrosive liquid. It decomposes rapidly in moist air and has a boiling point of about 168°F (75°C). PCl3 is corrosive to skin and tissue and reacts with water to form hydrochloric acid. The TLV is 0.2 ppm, and the IDLH is 50 ppm in air. The four-digit UN identification number is 1809. The NFPA 704 designation is health 4, flammability 0, and reactivity 2. The white section at the bottom of the diamond contains a W with a slash through it, indicating water reactivity. The primary uses are in the manufacture of organophosphate pesticides, gasoline additives, and dyestuffs; as a chlorinating agent; as a catalyst; and in textile finishing. Corrosives in contact with a poison may produce poison gases as the poison decomposes. In responding to an incident involving corrosives, the toxicity of the vapors could be much more of a concern for personnel than the corrosivity. When acids come in contact with cyanide, hydrogen cyanide gas, which is highly toxic, with a TLV of 10 ppm in air, is produced.
Chemical Properties
Phosphorus trichloride is a colorless to yellow, fuming liquid. Odor like hydrochloric acid.
Physical properties
Colorless fuming liquid; pungent odor; refractive index 1.516 at 14°C; density 1.574g/mL at 21°C; boils at 76°C; freezes at -112°C; decomposes in water; soluble in benzene, carbon disulfide, ether and chloroform and other halogenated organic solvents.
Uses
Phosphorus trichloride is an important intermediate in the production of insecticides, herbicides, and organophosphorus pesticides as well as other chemicals such as phosphoryl chloride, phosphorus pentachloride, thiophosphoryl chloride, and phosphonic acid. It is also used in the production of synthetic surfactants, phosphites, gasoline additives, flame retardants, silver polish, and producing iridescent metallic deposits.
Uses
Phosphorus trichloride is used as a chlorinating agent; as an intermediate in making gasoline additives, dyes, surfactants, and pesticides; in the manufacture of phosphorus pentachloride and phosphorus oxychloride; and as a catalyst.
Definition
ChEBI: Phosphorus trichloride is a phosphorus halide with formula Cl3P.
Production Methods
Phosphorus trichloride (PCl3) is made by reacting yellow phosphorus with chlorine and is used in chemical manufacturing. It hydrolyzes to phosphoric acid and hydrochloric acid.
Reactivity Profile
Phosphorus trichloride is a strong reducing agent that may ignite combustible organic materials upon contact. May generate flammable and potentially explosive gaseous hydrogen upon contact with many common metals (except nickel and lead). Reactions with water are violent and produce heat and flashes of fire [AAR, 1999]. Gives intensely exothermic reactions with iodine monochloride [Mellor 2, Supp. 1:502. 1956]. Several laboratory explosions have been reported arising from mixtures with acetic acid, along with other acids, sulfuric acid and derivatives, carboxylic acids, etc. These have been ascribed to poor heat control allowing the formation of phosphine [J. Am. Chem. Soc. 60:488. 1938]. Ignites when mixed with hydroxylamine [Mellor 8:290. 1946-47]. Causes an explosion on contact with nitric acid [Comp. Rend. 28:86]. Phosphorus trichloride is incompatible with many common oxidants such as: sodium peroxide, fluorine, chromyl chloride, iodine chloride, to name a few. Isopropanol can react with PCl3, forming toxic HCl gas. (Logsdon, John E., Richard A. Loke., "Isopropyl Alcohol." Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 1996.)
Hazard
Phosphorus trichloride is highly corrosive. Its vapors are an irritant to mucous membranes. Chronic exposure to its vapors can cause bronchitis. It reacts violently with water and explodes in contact with acetic and nitric acids, and several other substances (Patnaik. P. 1999. A Comprehensive Guide to the Hazardous Properties of Chemical Substances, 2nd. Ed. New York: John Wiley & Sons).
Health Hazard
Phosphorus trichloride is highly toxic; it may cause death or permanent injury. Contact is highly irritating to the skin, eyes, and mucous membranes, and the material is an irritant through oral and inhalation exposure.
Fire Hazard
Phosphorus trichloride will react violently with water, producing heat and toxic and corrosive fumes. When heated to decomposition, Phosphorus trichloride emits highly toxic fumes of chlorides and phosphorus oxides. Phosphorus trichloride may ignite other combustible materials. Reacts violently with water. Reacts explosively with acetic acid, aluminum, chromyl chloride, diallylphosphite and allyl alcohol, dimethyl sulfoxide, fluorine, hydroxylamine, iodine monochloride, lead dioxide, nitric acid, nitrous acid, organic matter, potassium, and sodium. Avoid contact with water, steam, or acids. Hazardous polymerization may not occur.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion and inhalation. A corrosive irritant to skin, eyes (at 2 ppm), and mucous membranes. Potentially explosive reaction with chlorobenzene + sodtum, hethyl sulfoxide, molten sodmm, chromyl chloride, nitric acid, sodium peroxide, oxygen (above 100℃), tetravinyl lead. Reacts with carboxylic acids (e.g., acetic acid) to form violently unstable products. Violent reaction or ignition with Al, chromium pentafluoride, dtallyl phosphite + allyl alcohol, F2, hexa fluoroisoprop ylideneaminolithium, hydroxylamine, iodine chloride, PbO2, HNO2, organic matter, potassium, selenium dioxide, sulfur acids (e.g., sulfuric acid, fluorosulfuric acid, oleum). Violent reaction with water evolves hydrogen chloride and diphosphane gas, that then ignite. Incompatible with metals or oxidants. Wdl react with water, steam, or acids to produce heat and toxic and corrosive fumes; can react with oxidzing materials. To fight fire, use CO2, dry chemical. Used as a chlorinating agent, catalyst, and chemical intermedtate. Dangerous; when heated to decomposition it emits highly toxic fumes of Cland POx.
Shipping
UN1809 Phosphorous trichloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Inhalation Zone B.
Purification Methods
Heat it under reflux to expel dissolved HCl, then distil it. It has been further purified by vacuum fractionation several times through a -45o trap into a receiver at -78o. [Forbes Inorg Synth II 145 1946.] HARMFUL VAPOURS.
Incompatibilities
Phosphorus trichloride is a strong reducing Violent reaction with water, producing heat and hydrochloric and phosphorous acids. Violent reaction with hydrides, alcohols, phenols and bases; water, when in contact with combustible organics; chemically active metals: sodium, potassium, aluminum; strong sulfuric or nitric acid. Attacks most metals except nickel and lead; may generate flammable hydrogen gas on contact with metals. Attacks plastics, rubber, and coatings.
Waste Disposal
Decompose with water, forming phosphoric and hydrochloric acids. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring, then flush to sewer with large volumes of water.
Phosphorus trichloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| HUAIAN DCHEN CHEM CO LTD | +86-57-80696996 +86-19816093969 | dchenchem@gmail.com | China | 16 | 58 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1001 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23541 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7832 | 58 |
| Yangzhou Model Electronic Materials Co., Ltd. | +8613761402923 | sales@modeltemol.com | CHINA | 571 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 2904 | 58 |
Related articles
- Phosphorus trichloride: Chemical properties, uses and toxicity
- Phosphorus trichloride (PCl3) is a colorless or slightly yellowish fuming liquid with a hydrochloric acid odor. It is soluble ....
- Dec 16,2024
- Why is PCl3 a polar molecule?
- PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electr....
- Dec 22,2023
- PCl3 Lewis structure: Drawing, Hybridisation, Geometry
- The Lewis structure of PCl3 consists of a central phosphorus atom (P) and three external chlorine atoms (Cl).
- Dec 6,2023
View Lastest Price from Phosphorus trichloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2022-04-07 | Phosphorus trichloride
7719-12-2
|
US $100.00 / bag | 1bag | 99 | 500 | Shanxi Naipu Import and Export Co.,Ltd |
-

- Phosphorus trichloride
7719-12-2
- US $100.00 / bag
- 99
- Shanxi Naipu Import and Export Co.,Ltd