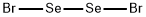SELENIUM BROMIDE
- CAS No.
- 7789-52-8
- Chemical Name:
- SELENIUM BROMIDE
- Synonyms
- 12049-00-2;SELENIUM BROMIDE;Einecs 232-173-5;selenium(I) bromide;Diselenium dibromide;SELENIUM MONOBROMIDE;SELENIUM BROMIDE 99%;seleniumbromide(se2br2)
- CBNumber:
- CB5319710
- Molecular Formula:
- Br2Se2
- Molecular Weight:
- 317.75
- MDL Number:
- MFCD00016326
- MOL File:
- 7789-52-8.mol
| Boiling point | 227, decomposes [KIR82] |
|---|---|
| Density | d415 3.604 |
| solubility | reacts with H2O; soluble in CS2, chloroform |
| form | red liquid |
| color | red |
| Water Solubility | decomposes in H2O; soluble chloroform, ethyl bromide, carbon disulfide [MER06] |
| FDA UNII | 678YXJ4HM6 |
| EPA Substance Registry System | Selenium bromide (Se2Br2) (7789-52-8) |
SELENIUM BROMIDE Chemical Properties,Uses,Production
Chemical Properties
Dark red, almost black, oily liquid with an unpleasant odour; hygroscopic; rapidly liberates Br2 in air, simultaneously separating Se. Partially decomposed on heating; first, some Br2 escapes, then some SeBr4 sublimes, and between 225 and 230°C, a part of the Se2Br2 boils without decomposition, leaving a residue of Se. In water, Se2Br2 sinks to the bottom in oily drops and gradually decomposes into Se, SeO2 and HBr, soluble in CS2 and CHCl3.
Production Methods
A round-bottom flask, equipped with a separatory funnel and gas outlet tube connected to a P2O5 drying tube, is filled with a suspension of 20 g of pure powdered Se in 50 ml of dry CS2; 20 g of pure Br2 is then slowly added from the separatory funnel. If the flask is occasionally shaken, the reaction is soon complete. A reddish-brown solution is formed, from which the CS2 is evaporated in a vacuum as rapidly as possible. The product is deep-red, pure Se2Br2.
Synthesis
A round-bottom flask, equipped with a separatory funnel and gas outlet tube connected to a P2O5 drying tube, is filled with a suspension of 20 g. of pure powdered Se in 50 ml. of dry CS2; 20 g. of pure Br2 is then slowly added from the separatory funnel. If the flask is occasionally shaken, the reaction is soon complete. A reddish-brown solution is formed, from which the CS2 is evaporated in vacuum as rapidly as possible. The product is deep-red, pure Se2Br2 .
SELENIUM BROMIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35426 | 58 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12338 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | 1046@dideu.com | China | 10011 | 58 |
| Jiangsu Raien Environmental Protection Technology Co., Ltd | 0513-66814573 16651336298 | 16651336298@163.com | China | 3000 | 58 |