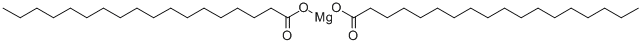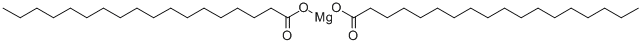Magnesium stearate
- CAS No.
- 557-04-0
- Chemical Name:
- Magnesium stearate
- Synonyms
- MAGNESIUM OCTADECANOATE;stearatedemagnesium;Magnesium Stearate Bp;C4d-A;Dolomol;Anti-C4d-A;硬脂酸镁生产厂家价格;petracmg20nf;nesium stearate;MAGENSIUM STEARATE
- CBNumber:
- CB5330900
- Molecular Formula:
- C36H70MgO4
- Molecular Weight:
- 591.24
- MDL Number:
- MFCD00036391
- MOL File:
- 557-04-0.mol
- MSDS File:
- SDS
| Melting point | 200 °C (lit.) |
|---|---|
| Density | 1.028g/cm3 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | alcohol: insoluble |
| form | Fine Powder |
| color | White |
| PH | 7 (H2O) (slurry) |
| Odor | wh. soft oily powd., tasteless, odorless |
| Water Solubility | Insoluble |
| Merck | 14,5690 |
| BRN | 3919702 |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 3 mg/m3 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | DRJIJXNWSSRTTE-UHFFFAOYSA-M |
| LogP | 8.216 (est) |
| CAS DataBase Reference | 557-04-0(CAS DataBase Reference) |
| FDA 21 CFR | 184.1440; 173.340; 175.300; 181.29 |
| Substances Added to Food (formerly EAFUS) | MAGNESIUM STEARATE |
| SCOGS (Select Committee on GRAS Substances) | Magnesium stearate |
| EWG's Food Scores | 1 |
| FDA UNII | 70097M6I30 |
| EPA Substance Registry System | Magnesium stearate (557-04-0) |
| Cosmetics Info | Magnesium Stearate |
Magnesium stearate price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 26454 | Magnesium stearate puriss., meets analytical specification of Ph. Eur., BP, ≥90% stearic and palmitic acid basis, ≥40% stearic acid basis (GC), 4.0-5.0% Mg basis (calc on dry sub.) | 557-04-0 | 1kg | $50.7 | 2024-03-01 | Buy |
| TCI Chemical | S0238 | Magnesium(II) Stearate | 557-04-0 | 25g | $16 | 2024-03-01 | Buy |
| TCI Chemical | S0238 | Magnesium(II) Stearate | 557-04-0 | 250g | $34 | 2024-03-01 | Buy |
| Alfa Aesar | A16244 | Magnesium stearate | 557-04-0 | 250g | $54 | 2024-03-01 | Buy |
| Alfa Aesar | A16244 | Magnesium stearate | 557-04-0 | 1000g | $164 | 2024-03-01 | Buy |
Magnesium stearate Chemical Properties,Uses,Production
Surfactant
Magnesium stearate is a kind of fatty acid salt type anionic surfactant with its appearance being white powder with slight special smell and creamy feeling. It can be soluble in hot aliphatic hydrocarbons, hot arene and hot grease but insoluble in alcohol and water with being decomposed into stearic acid and corresponding magnesium salts in case of acid. Magnesium stearate has an excellent adhesion property to the skin with excellent lubrication property. It can be applied to powder products in cosmetics and can improve adhesion and lubrication.

Magnesium stearate can be used as PVC heating stabilizers with the stability performance being similar to calcium stearate and can be combined with zinc or calcium soaps for being applied to food packaging material but without very wide application.
Magnesium stearate can be used as a mold releasing agent of plastic products, face powder of cosmetics, the raw material of skin ointment, the powder molding tablet of pharmaceutical tablets and translucent flatting agent of paint.
Laboratory, through the replacement reaction of sodium stearate and magnesium sulfate, is able to get finished product of magnesium stearate and can also apply the combination reaction between edible solid organic acids (stearic acid, palmitic acid) mixture and magnesium oxide compounds and further refinement to make it.
Chemical Properties
Magnesium stearate is a fatty acid, salt-type anionic surfactant with its appearance being white powder with a creamy feeling. It is a compound of magnesium with a mixture of solid organic acids obtained from edible sources and consists chiefly of variable proportions of magnesium stearate and magnesium palmitate.
It appears as bright white soft powder with the industrial products containing a small amount of oleic acid and 7% magnesium oxide and is odorless and tasteless. It is slightly soluble in water and soluble in hot ethanol.
Uses
Magnesium stearate has been widely used for many decades in the food industry as an emulsifier, binder and thickener, as well as an anticaking, lubricant, release, and antifoaming agent. It is present in many food supplements, confectionery, chewing gum, herbs and spices, and baking ingredients. Magnesium stearate is also commonly used as an inactive ingredient in the production of pharmaceutical tablets, capsules and powders. The main reason for its good lubricating properties is its hydrophobic nature and an ability to reduce friction between tablets and die wall during the ejection process.
Magnesium stearate can be regarded as being non-toxic, the United States, Germany and Japan allow it to be applied to products being contact with food. However, it doesn’t have wide application to be applied as PVC heat stabilizers. In China and some other countries Pharmacopoeia, there are always records of this specie. The pharmacopeia has also made provisions on the magnesium content, moisture, heavy metals, iron, sulfate and chloride content.
Pharmaceutical Applications
Magnesium stearate is the magnesium salt of stearic acid that possess lubricating properties and hence prevents ingredients from sticking to manufacturing equipment during the compression of chemical powders into solid tablets. It has been used as a tablet and capsule lubricant. It has also been used for preparing microcapsules. Dry coating of drugs with magnesium stearate leads to flow improvement, flow-aid and lubrication effects, tabletability as well as non-inhibited dissolution rate.
Production method
Magnesium stearate is produced by the reaction of sodium stearate with magnesium salts or by treating magnesium oxide with stearic acid (Nora 2005).
Magnesium stearate can be produced through the following procedure: first get the sodium stearate through the saponification between stearic acid and sodium; then the sodium stearate has double decomposition reaction with magnesium sulfate to get the finished product. Stearic acid and water was added to the reaction pot and heated to 85 ℃, stirring to dissolve, slowly add them to the sodium hydroxide solution preheated to 75 ℃. After the saponification reaction was completed, the temperature was controlled at 72 ℃ and slowly added to the magnesium sulfate solution preheated to 55 ℃ upon stirring. After metathesis, apply centrifuge to remove the water. The filtering cake was washed with water until sulfate ion requirement is met, then dry, apply air drying, sifting to obtain the finished products with the yield of stearic acid being 100%.
It is produced through the combination reaction between magnesium oxide and food grade solid mixed fatty acids (mainly stearic acid) and further refinement.
Toxicity
Magnesium stearate is considered to be non-toxic, and is Generally Recognized As Safe (GRAS) by the U.S. Food and Drug Administration (FDA). It is approved for use in food and dietary supplements as a lubricant and release agent, emulsifier, binder, thickener, anticaking and anti-foaming agent. In addition to the United States, it is accepted as a safe food additive in Europe, the UK, and Canada. A specification for magnesium stearate is also included in the Food Chemicals Codex (FCC), a collection of internationally recognized standards for the purity and identity of food ingredients. According to the FDA, there is no evidence to suggest that magnesium stearate causes adverse effects when used “at levels that are now current and in the manner now practiced, or which might reasonably be expected in the future.” Animal research shows that orally-administered magnesium stearate is non-toxic far beyond the commonly used amounts. Additionally, as recently as 2015, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) conducted a safety assessment of magnesium stearate and found no concerns regarding its continued use or safety.
Description
Magnesium stearate, also called octa decanoic acid, magnesium salt, is a white substance, powder which becomes solid at room temperature. It has the chemical formula Mg(C18H35O2)2. It is a salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate melts at about 120 °C, is not soluble in water, and is generally considered safe for human consumption at levels below 2500 mg/kg per day.In 1979, the FDA's Subcommittee on GRAS (generally recognized as safe) Substances (SCOGS) reported, "There is no evidence in the available information on ... magnesium stearate ... that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when they are used at levels that are now current and in the manner now practiced, or which might reasonably be expected in the future.".
Chemical Properties
Magnesium stearate is a compound of magnesium with a mixture of solid organic acids obtained from edible sources and consists chiefly of variable proportions of magnesium stearate and magnesium palmitate. It occurs as a fine, white, bulky powder having a faint, characteristic odor. It is unctuous and is free from grittiness. It is insoluble in water, in alcohol, and in ether. It conforms to the regulations of the U.S. Food and Drug Administration pertaining to specifications for salts of fatty acids derived from edible fat sources.
Chemical Properties
Magnesium stearate is a very fine, light white, precipitated or milled, impalpable powder of low bulk density, having a faint odor of stearic acid and a characteristic taste. The powder is greasy to the touch and readily adheres to the skin. nonflammable used in baby dusting powder(s) and as tablet lubricant.
Uses
Magnesium Stearate is the magnesium salt of stearic acid which functions as a lubricant, binder, emulsifier, and anticaking agent. it is a white powder that is insoluble in water. it is used as a lubricant or die release in tableting pressed candies and is also used in sugar- less gum and mints.
Uses
Magnesium stearate is often used as a anti-adherent in the manufacture of medical tablets, capsules and powders. In this regard, the substance is also useful, because it has lubricating properties, preventing ingredients from sticking to manufacturing equipment during the compression of chemical powders into solid tablets; magnesium stearate is the most commonly used lubricant for tablets. Studies have shown that magnesium stearate may affect the release time of the active ingredients in tablets, etc., but not that it reduces the overall bioavailability of those ingredients. As a food additive or pharmaceutical excipient, its E number is E470b.
Magnesium stearate is also used to bind sugar in hard candies like mints, and is a common ingredient in baby formulas. In pure powder form, the substance can be a dust explosion hazard, although this issue is effectively insignificant beyond the manufacturing plants using it.
Magnesium stearate is manufactured from both animal and vegetable oils. Some nutritional supplements specify that the magnesium stearate used is sourced from vegetables.
Magnesium stearate is a major component of "bathtub rings." When produced by soap and hard water, magnesium stearate and calcium stearate both form a white solid insoluble in water, and are collectively known as "soap scum.".
Uses
widely used in the fields of the plastic, mold-releasing agent for tablets (need meeting the medicine criterion), emulsifying agents of cosmetics. It also can conjugate with Ca Soap as stabilizer of PVC.
Preparation
Magnesium stearate is created by the reaction of sodium stearate with magnesium sulfate.
Production Methods
Magnesium stearate is prepared either by the interaction of aqueous solutions of magnesium chloride with sodium stearate or by the interaction of magnesium oxide, hydroxide, or carbonate with stearic acid at elevated temperatures.
Definition
Mg(C18H35O2)2 or with one H2O. Technical grade contains small amounts of the oleate and 7% magnesium oxide, MgO.
General Description
Magnesium stearate (Mg-St) is the magnesium salt of stearic acid. Its anhydrate, dihydrate and trihydrate forms have been prepared. The tabletting of the blends of magnesium stearate and lactose granules has been described. The influence of mixing time on hardness, disintegration time and ejection force on the compressed tablets was examined. Mg-St is widely used lubricant in pharmaceutical industry. It also plays a role in delaying the process of dissolution. Its detection in tablets by laser-induced breakdown spectroscopy has been proposed.
Pharmaceutical Applications
Magnesium stearate is widely used in cosmetics, foods, and pharmaceutical formulations. It is primarily used as a lubricant in capsule and tablet manufacture at concentrations between 0.25% and 5.0% w/w. It is also used in barrier creams.
Biochem/physiol Actions
Magnesium stearate is the magnesium salt of stearic acid that possess lubricating properties and hence prevents ingredients from sticking to manufacturing equipment during the compression of chemical powders into solid tablets. Dry coating of drugs with magnesium stearate leads to flow improvement, flow-aid and lubrication effects, tabletability as well as non-inhibited dissolution rate.
Safety Profile
Slightly toxic by ingestion. Spontaneously combustible. When heated to decomposition it emits acrid smoke and toxic fumes.
Safety
Magnesium stearate is widely used as a pharmaceutical excipient
and is generally regarded as being nontoxic following oral
administration. However, oral consumption of large quantities
may produce a laxative effect or mucosal irritation.
No toxicity information is available relating to normal routes of
occupational exposure. Limits for heavy metals in magnesium
stearate have been evaluated in terms of magnesium stearate worstcase
daily intake and heavy metal composition.
Toxicity assessments of magnesium stearate in rats have
indicated that it is not irritating to the skin, and is nontoxic when
administered orally or inhaled.
Magnesium stearate has not been shown to be carcinogenic
when implanted into the bladder of mice.
LD50 (rat, inhalation): >2 mg/L
LD50 (rat, oral): >10 g/kg
storage
Magnesium stearate is stable and should be stored in a well-closed container in a cool, dry place.
Incompatibilities
Incompatible with strong acids, alkalis, and iron salts. Avoid mixing with strong oxidizing materials. Magnesium stearate cannot be used in products containing aspirin, some vitamins, and most alkaloidal salts.
Regulatory Status
GRAS listed. Accepted as a food additive in the USA and UK. Included in the FDA Inactive Ingredients Database (oral capsules, powders, and tablets; buccal and vaginal tablets; topical preparapreparations; intravitreal implants and injections). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. Listed on the US TSCA inventory.
Magnesium stearate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 | marketing1@neostarunited.com | China | 8838 | 58 |
| Watson Biotechnology Co.,Ltd | +86-18186686046 +86-18186686046 | sales01@watsonbiotech.cn | China | 5822 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Changzhou Rokechem Technology Co., Ltd. | 18758118018 | sales001@rokechem.com | China | 255 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18154 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Guangzhou Tengyue Chemical Co., Ltd. | +86-86-18148706580 +8618826483838 | evan@tyvovo.com | China | 148 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Shandong Juchuang Chemical Co., LTD | +undefined15030412209 | admin@juchuangchem.com | China | 387 | 58 |
Related articles
- Magnesium Stearate: Applications in Pharmaceutical Industry and Health Concerns
- Magnesium stearate, especially as MgSt-SLN nanoparticles, improves tablet quality but excessive intake may pose health risks, ....
- Jun 24,2024
- Introduction of Magnesium Stearate
- Magnesium distearate is a magnesium salt. Magnesium stearate is a white, dry, easy, sand free and smooth powder, non-toxic,
- Jan 12,2022
- Magnesium stearate-Physical and Chemical Properties
- Magnesium stearate is a white, non-sandy fine powder; and has a slight odor with a slippery feel when it comes in contact with....
- Jan 9,2020
View Lastest Price from Magnesium stearate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Magnesium stearate
557-04-0
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2024-11-25 | Magnesium stearate
557-04-0
|
US $1000.00-700.00 / ton | 1ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-25 | Magnesium stearate
557-04-0
|
US $6.00 / KG | 1KG | 99% | 20TONS | Hebei Longbang Technology Co., Ltd |
-

- Magnesium stearate
557-04-0
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd
-

- Magnesium stearate
557-04-0
- US $1000.00-700.00 / ton
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Magnesium stearate
557-04-0
- US $6.00 / KG
- 99%
- Hebei Longbang Technology Co., Ltd