Ferrous sulfide
- CAS No.
- 1317-37-9
- Chemical Name:
- Ferrous sulfide
- Synonyms
- FeS48;ci77540;Thioxoiron;ironsulfuret;iron sulphide;Iron(II)ulfide;IRON(+2)SULFIDE;Iron(Ⅱ) sulfide;FERROUS SULFIDE;IRON(II) SULFIDE
- CBNumber:
- CB5452626
- Molecular Formula:
- FeS
Lewis structure

- Molecular Weight:
- 87.91
- MDL Number:
- MFCD00011013
- MOL File:
- 1317-37-9.mol
- MSDS File:
- SDS
| Melting point | 1195 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | decomposes [HAW93] | ||||||||||||||
| Density | 4.84 g/mL at 25 °C(lit.) | ||||||||||||||
| vapor pressure | 0Pa at 25℃ | ||||||||||||||
| storage temp. | no restrictions. | ||||||||||||||
| solubility | insoluble in H2O; reacts with acid solutions | ||||||||||||||
| form | Sticks | ||||||||||||||
| color | Grayish-black | ||||||||||||||
| Specific Gravity | 4.84 | ||||||||||||||
| Odor | Odorless | ||||||||||||||
| Water Solubility | Soluble in water(0.0062g/L ), in acids with evolution of hydrogen sulfide. Insoluble in nitric acid. | ||||||||||||||
| Crystal Structure | NiAs type | ||||||||||||||
| Sensitive | Moisture Sensitive | ||||||||||||||
| Merck | 14,4058 | ||||||||||||||
| crystal system | Six sides | ||||||||||||||
| Solubility Product Constant (Ksp) | pKsp: 17.2 | ||||||||||||||
| Space group | P63/mmc | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Stability | Stable. Incompatible with strong acids, strong bases, metal oxides. Avoid moisture. | ||||||||||||||
| CAS DataBase Reference | 1317-37-9(CAS DataBase Reference) | ||||||||||||||
| FDA UNII | TH5J4TUX6S | ||||||||||||||
| EPA Substance Registry System | Ferrous sulfide (1317-37-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P280-P302+P352-P304+P340 | |||||||||
| Hazard Codes | N | |||||||||
| Risk Statements | 31-50 | |||||||||
| Safety Statements | 60-61-50 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 13 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28309011 | |||||||||
| NFPA 704 |
|
Ferrous sulfide price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 268704 | Iron(II) sulfide technical grade | 1317-37-9 | 250g | $81.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 12363 | Iron(II) sulfide sticks (thin) | 1317-37-9 | 1kg | $143 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.03956 | Iron(II) sulfide sticks ? ~ 1 cm | 1317-37-9 | 1kg | $69 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.03956 | Iron(II) sulfide sticks ? ~ 1 cm | 1317-37-9 | 25kg | $820 | 2024-03-01 | Buy |
| Alfa Aesar | 014024 | Iron(II) sulfide, 99.9% (metals basis) | 1317-37-9 | 10g | $70.1 | 2024-03-01 | Buy |
Ferrous sulfide Chemical Properties,Uses,Production
Uses
Iron(II) sulfide occurs in nature as the minerals magnetkies, troillite and pyrrhotine. The most important application of this compound is in Kipp’s apparatus as a source for laboratory preparation of hydrogen sulfide. It also is used in paints, pigments, and ceramics and lubricant coatings.
Preparation
Iron(II) sulfide may be synthesized from the elements but the product is contaminated with iron. The reaction is exothermic and the heat of reaction melts iron. Pure sulfide may be obtained by using a slight excess of sulfur: the excess then is distilled off.
The compound also may be precipitated by treating an aqueous solution of an alkali metal sulfide with that of iron(II) chloride or any iron(II) salt solution:
S2– (aq) + Fe2+ (aq) → FeS(s)
Another method of preparation involves passing a mixture of hydrogen sulfide and hydrogen over iron(III) oxide at about 1,000°C:
Fe2O3 + 2H2S + H2 → 2FeS + 3H2O
Reactions
Iron(II) sulfide reacts with acids evolving hydrogen sulfide:
FeS + 2HCl → H2S + FeCl2
The compound is readily oxidized under moist condition by action of air, forming triiron tetroxide and elemental sulfur:
3FeS + 2O2 → Fe3O4 + 3S
The above reaction is exothermic.
Iron(II) sulfide decomposes to its elements when heated above 1,100°C:
FeS → Fe + S
When heated with boiled water, it generates sulfuric acid and hydrogen:
4FeS + 8H2O + 7O2 → 4H2SO4 + 4H2 + Fe2O3
Chemical Properties
grey to brown-black lumps or powder
Physical properties
Colorless hexagonal or tetragonal crystals; density 4.7g/cm3; melts at 1188°C; insoluble in water; soluble in acids (reacts).
Uses
Iron(II) sulfide is used to generate hydrogen sulfide instantaneously by reacting with hydrochloric acid. In laboratory, Kipp?s apparatus is utilized for this purpose. It is used as a re-sulphurizing and alloying agent, as a reducing agent to remove heavy metal impurities from phosphoric acid. It is used to control hydrogen embrittlement in alloy and stainless steel industries.
Uses
Ferrous sulfide can be used as a laboratory source of H2S; in the ceramic industry; as a paint pigment; in anodes; in lubricant coatings.
Flammability and Explosibility
Non flammable
Ferrous sulfide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20288 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21639 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29815 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34553 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2737 | 58 |
| Alfa Chemistry | Info@alfa-chemistry.com | United States | 24072 | 58 |
View Lastest Price from Ferrous sulfide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
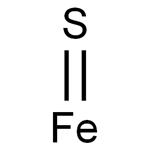 |
2024-09-04 | Ferrous sulfide
1317-37-9
|
US $0.00-0.00 / KG | 1KG | 99.0% | 10000KG | Shaanxi Dideu Medichem Co. Ltd | |
 |
2024-03-29 | Ferrous sulfide
1317-37-9
|
US $6.00-1.00 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2020-01-13 | Ferrous sulfide
1317-37-9
|
US $1.00 / KG | 1KG | 98%-99.9% | 200kg | Career Henan Chemical Co |
-
- Ferrous sulfide
1317-37-9
- US $0.00-0.00 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
-

- Ferrous sulfide
1317-37-9
- US $6.00-1.00 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Ferrous sulfide
1317-37-9
- US $1.00 / KG
- 98%-99.9%
- Career Henan Chemical Co





