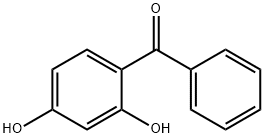
2,4-Dihydroxybenzophenone
- CAS No.
- 131-56-6
- Chemical Name:
- 2,4-Dihydroxybenzophenone
- Synonyms
- BP-1;UV-0;BENZOPHENONE-1;Uv 12;BENZORESORCINOL;uv12;4-BENZORESORCINOL;hhb;uf1;Uf 1
- CBNumber:
- CB5475441
- Molecular Formula:
- C13H10O3
- Molecular Weight:
- 214.22
- MDL Number:
- MFCD00002277
- MOL File:
- 131-56-6.mol
- MSDS File:
- SDS
| Melting point | 144.5-147 °C(lit.) |
|---|---|
| Boiling point | 194 °C (1 mmHg) |
| Density | 1,32 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5090 (estimate) |
| Flash point | 125 °C |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly, Heated), Ethanol (Slightly, Heated) |
| pka | 7.72±0.35(Predicted) |
| form | Crystalline Powder |
| color | Yellow |
| PH | 5 (10g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Merck | 14,1106 |
| BRN | 1311566 |
| InChIKey | ZXDDPOHVAMWLBH-UHFFFAOYSA-N |
| LogP | 2.964 at 25℃ |
| CAS DataBase Reference | 131-56-6(CAS DataBase Reference) |
| EWG's Food Scores | 4 |
| FDA UNII | LJ54R4Z029 |
| NIST Chemistry Reference | Methanone, (2,4-dihydroxyphenyl)phenyl-(131-56-6) |
| EPA Substance Registry System | Methanone, (2,4-dihydroxyphenyl)phenyl- (131-56-6) |
| Cosmetics Info | Benzophenone-1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H361fd-H411 | |||||||||
| Precautionary statements | P202-P264-P273-P280-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xi,T | |||||||||
| Risk Statements | 36-36/37/38-61 | |||||||||
| Safety Statements | 26-37/39-45-53 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DJ0700000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29145000 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg | |||||||||
| NFPA 704 |
|
2,4-Dihydroxybenzophenone price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SRP5162 | 4EBP1, His tagged human recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution | 185703-54-2 | 50μG | $349 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.18652 | 2,4-Dihydroxybenzophenone for synthesis | 131-56-6 | 250g | $105 | 2024-03-01 | Buy |
| Sigma-Aldrich | 126217 | 2,4-Dihydroxybenzophenone 99% | 131-56-6 | 100g | $45.3 | 2024-03-01 | Buy |
| TCI Chemical | D0573 | 2,4-Dihydroxybenzophenone >98.0%(GC)(T) | 131-56-6 | 25g | $20 | 2024-03-01 | Buy |
| TCI Chemical | D0573 | 2,4-Dihydroxybenzophenone >98.0%(GC)(T) | 131-56-6 | 500g | $153 | 2024-03-01 | Buy |
2,4-Dihydroxybenzophenone Chemical Properties,Uses,Production
pollution
Benzophenones (BPs), as UV filters, are widely utilized in personal care products such as sunscreen, body lotion and plastic products. 2,4-Dihydroxybenzophenone (BP-1) is a representative type of BP that has attracted wide attention owing to the high residues in the aquatic environment. According to previous studies, this compound can enter the aquatic environment through recreational water activities or incomplete removal in wastewater treatment plants (WWTPs). For example, BP-1 has been widely detected in seawater, groundwater, river water and WWTP effluents in Spain at concentrations ranging from 4.2 ng/L to 722.0 ng/L. More seriously, because of extensive human exposure through plastic products and sunscreen cosmetics, BP-1 has been detected in human urine, blood and milk[1].
Description
2,4-Dihydroxybenzophenone (DHP) is an organic compound derived from Garcinia xanthochymus, but there have been no reports on its biochemical functions and bioavailability. 2,4-dihydroxybenzophenone has been prepared by reacting resorcinol with benzoyl chloride or benzoic acid under anhydrous conditions in the presence of Friedel-Crafts catalysts such as AlCl3 or ZnCl2[1].
Chemical Properties
yellow crystalline powder
Uses
Novel mordent and disperse azo dyes were prepared by the coupling of various diazo solutions of aromatic amines with 2, 4-dihydroxybenzophenone. The ultraviolet absorbing monomers were synthesized by reaction of 2, 4-dihydroxybenzophenone with glycidyl acrylate and glycidyl methacrylate. Two new polymerizable stabilizers, 4-benzoyl-2-(α-piperidino-2-chlorobenzyl)-3-hydroxyphenyl acrylate (BPBHA) and corresponding methacrylate (BPBHMA), were synthesized and characterized from 2,4-Dihydroxybenzophenone. a polymerizable UV-stabilizer, 2-hydroxy-4-(3-methacryloxy-2-hydroxylpropoxy) benzophenone (BPMA), was synthesized using 2, 4-dihydroxybenzophenone (UV-0) and glycidyl methacrylate (GMA).
Uses
Ultraviolet light absorber, especially in paints and plastics.
Uses
2,4-Dihydroxybenzophenone is an metabolite of Benzophenone (B204980), an compound used in the manufacturing of antihistamines, hypnotics and insecticides.
Definition
ChEBI: 2,4-Dihydroxybenzophenone is a member of benzophenones.
Preparation
Obtained by condensation of benzanilide with resorcinol in the presence of zinc chloride and phosphorous oxychloride at 130–140° for 1 h (25%).
Biological Activity
2,4-Dihydroxybenzophenone has potential effects on the stimulation of osteoblast differentiation and mitigate PDS-induced osteoporosisthrough the activation of the β-catenin pathway. Moreover, 2,4-Dihydroxybenzophenone demonstrated a capacity to enhance the expression of crucial osteogenic markers, including RUNX2, OSX, and ALP[2].
Contact allergens
BZP-1 is used, for example, in paints, plastics, and nail varnishes.
Safety Profile
Poison by intravenous and intraperitoneal routes. Mildly toxic by ingestion. An eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Toxicology
As a persistent and bioaccumulative compound, 2,4-Dihydroxybenzophenone (BP-1) exhibits estrogenic activity or anti-androgenic activity in vitro, which could pose potential risks to the ecological environment and human health. At present, there are a large amount of reports that concentrated on the biotoxicity of BP-1, mainly focusing on its endocrine disrupting effects and genetic toxicity. Endocrine-toxicological studies have shown that the toxicity of BP-1 is five times that of bisphenol A (BPA)[2].
Purification Methods
Recrystallise it from MeOH. [Beilstein 8 IV 2442.]
References
[1] Mengting Zou . “Effective degradation of 2,4-dihydroxybenzophenone by zero–valent iron powder (Fe0)-activated persulfate in aqueous solution: Kinetic study, product identification and theoretical calculations.” Science of the Total Environment 771 (2021): Article 144743.
[2] Mirissa Hewage Dumindu Kavinda and & Gi-Young Kim*. “2,4′-Dihydroxybenzophenone Exerts Bone Formation and Antiosteoporotic Activity by Stimulating the β-Catenin Signaling Pathway.” ACS Pharmacology and Translational Science 7 2 (2024): 395–405.
2,4-Dihydroxybenzophenone Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Qingdao Truelight functional Material Technology Co., Ltd. | +86-13573844393 | songjames@truelightchem.com | China | 221 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18153 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Shengyang Water Conservancy Engineering Co., Ltd. | +8615373025980 | clara@hbshengyang.com | China | 874 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1177 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
View Lastest Price from 2,4-Dihydroxybenzophenone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-29 | 2,4-Dihydroxybenzophenone
131-56-6
|
US $0.00-0.00 / Kg/Drum | 1KG | 99%min | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-29 | UV ABSORBER UV-0
131-56-6
|
US $80.00 / kg | 1kg | 99 | 5000 | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-29 | UV Absorber Benzophenone-1 UV-0
131-56-6
|
US $120.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |
-

- 2,4-Dihydroxybenzophenone
131-56-6
- US $0.00-0.00 / Kg/Drum
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- UV ABSORBER UV-0
131-56-6
- US $80.00 / kg
- 99
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- UV Absorber Benzophenone-1 UV-0
131-56-6
- US $120.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd