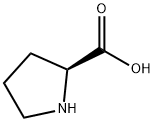
L-Proline
- CAS No.
- 147-85-3
- Chemical Name:
- L-Proline
- Synonyms
- PRO;H-PRO-OH;L-PRO;l-prolin;(S)-PYRROLIDINE-2-CARBOXYLIC ACID;2-pyrrolidinecarboxylic acid;L-Pro-OH;(2S)-pyrrolidine-2-carboxylic acid;L-Poline;(S)-Prolin
- CBNumber:
- CB6118196
- Molecular Formula:
- C5H9NO2
- Molecular Weight:
- 115.13
- MDL Number:
- MFCD00064318
- MOL File:
- 147-85-3.mol
- MSDS File:
- SDS
| Melting point | 228 °C (dec.) (lit.) |
|---|---|
| Boiling point | 215.41°C (rough estimate) |
| alpha | -85.5 º (c=4, H2O) |
| Density | 1.35 |
| vapor pressure | 0Pa at 25℃ |
| FEMA | 3319 | L-PROLINE |
| refractive index | -85 ° (C=4, H2O) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| pka | 1.95, 10.64(at 25℃) |
| color | White |
| Odor | at 100.00 %. odorless |
| PH | 6.0-7.0 (25℃, 1M in H2O) |
| optical activity | [α]20/D 85.0±1.0°, c = 5% in H2O |
| Odor Type | odorless |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| JECFA Number | 1425 |
| Merck | 14,7780 |
| BRN | 80810 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | ONIBWKKTOPOVIA-UHFFFAOYSA-N |
| LogP | -2.54 at 20℃ |
| Substances Added to Food (formerly EAFUS) | L-PROLINE |
| FDA 21 CFR | 172.320 |
| CAS DataBase Reference | 147-85-3(CAS DataBase Reference) |
| FDA UNII | 9DLQ4CIU6V |
| NIST Chemistry Reference | Proline(147-85-3) |
| EPA Substance Registry System | L-Proline (147-85-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-22 | |||||||||
| Safety Statements | 24/25-36/37/39-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TW3584000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29339990 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5110 mg/kg | |||||||||
| NFPA 704 |
|
L-Proline price More Price(87)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W331902 | L-Proline 99%, FCC, FG | 147-85-3 | 100g | $71 | 2024-03-01 | Buy |
| Sigma-Aldrich | W331902 | L-Proline 99%, FCC, FG | 147-85-3 | 1kg | $148 | 2024-03-01 | Buy |
| Sigma-Aldrich | W331902 | L-Proline 99%, FCC, FG | 147-85-3 | 5kg | $595 | 2024-03-01 | Buy |
| Sigma-Aldrich | P5607 | L-Proline from non-animal source, meets EP, USP testing specifications, suitable for cell culture | 147-85-3 | 25KG | $9360 | 2024-03-01 | Buy |
| Sigma-Aldrich | 81709 | L-Proline BioUltra, ≥99.5% (NT) | 147-85-3 | 10g | $74.5 | 2024-03-01 | Buy |
L-Proline Chemical Properties,Uses,Production
Chemical Properties
L-Proline, an amino acid, is colorless to white crystal or crystalline powder that has a slight, characteristic odor with a slightly sweet taste. It is soluble in water, insoluble in ethanol, diethyl ether and n-butanol, yellow in case of hydrated ninhydrin test solution, glacial acetic acid Red after acidification; pH=6.3, decomposition point is 220-222°C; specific optical rotation [α]20D-85° (0.5-2.0mg/ml, H2O), [α]20D-60.4° (0.5-2.0mg /ml, 5mol/LHCl). It is synthesized from L-glutamine and L-glutamate via L-ornithine in intestine, and from L-ornithine in liver. It is widely used as an ingredient in infusion and infant formula.
Occurrence
Reported found as a component in many proteins; also widely occurring as the free acid in natural products. A major constituent of collagen, the main fibrous protein found in bone, cartilage and other connective tissue.
Uses
L-Proline is an amino acid and precursor (with vitamin C) for collagen, the building block of the structure of tendons, ligaments, arteries, veins and muscles. It is important in wound healing.
Uses
L-Proline is used as asymmetric catalysts in organic synthesis and asymmetric aldol cyclization. It is involved in the Michael addition of dimethyl malonate to alfa-beta-unsaturated aldehydes. It is a precursor of hydroxyproline in collagen. It is an active component of collagen and involved in the proper functioning of joints and tendons. It finds uses in pharmaceutical, biotechnological applications due to its osmoprotectant property. Further, it is used with ninhydrin in the chromatography.
Preparation
Synthesis of L-proline: Using glutamic acid as a raw material, it is esterified with absolute ethanol under the catalysis of sulfuric acid, and triethanolamine is added to free the aminosulfate to obtain glutamic acid-δ-ethyl ester. The glutamic acid-δ-ethyl ester is then reduced with a metal reducing agent potassium borohydride to obtain crude proline, which is finally separated and purified to obtain crude L-proline.
Definition
ChEBI: L-proline is pyrrolidine in which the pro-S hydrogen at position 2 is substituted by a carboxylic acid group. L-Proline is the only one of the twenty DNA-encoded amino acids which has a secondary amino group alpha to the carboxyl group. It is an essential component of collagen and is important for proper functioning of joints and tendons. It also helps maintain and strengthen heart muscles. It has a role as a micronutrient, a nutraceutical, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a member of compatible osmolytes. It is a glutamine family amino acid, a proteinogenic amino acid, a proline and a L-alpha-amino acid. It is a conjugate base of a L-prolinium. It is a conjugate acid of a L-prolinate. It is an enantiomer of a D-proline. It is a tautomer of a L-proline zwitterion.
Biotechnological Production
Direct fermentation using analogue-resistant mutants of coryneform bacteria or Serratia marcescens is an economic production method. An isoleucine auxotrophic mutant of Brevibacterium flavum having resistance to sulfaguanidine and D,L-3,4-dehydroproline (DP) is able to accumulate 40 g/L L-proline. Brevibacterium flavum AP113 is claimed to produce 97.5 g/L L-proline; this mutant is characterized by isoleucine auxotrophy, resistance to DP, and osmotic pressure and incapable to degrade Lproline. A proline oxidase-less strain of Serratia marcescens, having resistance to DP, thiazoline-4-carboxylate and azetidine-2-carboxylate, overproduces 58.5 g/L L-proline into the culture medium. By amplification of the genes proA and proB in this type of regulatory mutant, a construct was obtained which yields 75 g/L L-proline.
benefits
L-proline is considered a non-essential amino acid as it can be synthesised from arginine via the urea cycle in liver, and from glutamine/glutamic acid in the intestinal epithelium. It has a number of beneficial properties including connective tissue strengthening, Stronger Connective Tissue, Decreased Risk Of Heart Disease, Maintenance Of Muscle Tissueand skin health.
General Description
L-Proline is a non-essential amino acid, which is a building block of proteins. Peptides bond to proline, making it a useful building block for proteins. It can be used as a cell culture media component for the commercial biomanufacturing of therapeutic recombinant proteins and monoclonal antibodies. L-Proline plays important roles in various biological processes. It is involved in the synthesis of collagen, which is one of the most abundant proteins in the human body and provides structural support to tissues such as skin, bone, cartilage, and tendons.
Biochem/physiol Actions
Proline is a cyclic, non-essential, hydrophobic amino acid. It is a proteinogenic amino acid which is crucial for primary metabolism. In peptide chains, proline residues confer structural constraints and enhance the susceptibility of proximal peptide bonds to protease activity.
Side effects
The only known side effects are reactions from taking too much L-proline, like all amino acids. It causes toxicity levels and amino acid imbalances in your body.
Purification Methods
A likely impurity is hydroxyproline. Purify L-proline via its picrate which is crystallised twice from water, then decomposed with 40% H2SO4. The picric acid is extracted with diethyl ether, the H2SO4 in solution is precipitated with Ba(OH)2, and the filtrate is evaporated. The residue is crystallised from hot absolute EtOH [Mellan & Hoover J Am Chem Soc 73 3879 1951] or EtOH/Et2O. Its solubility in H2O is >100%. It sublimes at 182-187o/0.3mm with 99.4% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. It is hygroscopic and is stored in a desiccator. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2178-2199 1961, Beilstein 22 III/IV 8, 22/1 V 31.]
Structure and conformation
L-proline, also known as L-pyrrolidine-2-carboxylic acid, is a neutral amino acid. Although proline is classified as an amino acid, it is strictly speaking an imino acid, since it contains an imino group (carbon-nitrogen double bond). Due to its cyclic pyrrolidine side chain it is classified as a nonpolar aliphatic amino acid.
L-Proline Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
| Maanshan Tiantai Biotechnology Co., Ltd. | +86-0555-8889890 +86-17356584060 | GM@tiantaibio-tech.com | China | 72 | 58 |
| Lewoo Pharmatech(Shanghai)Co.,Ltd | +86-15800805830 +86-15800805830 | miao@lewoopharma.com.cn | China | 184 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +86-15350851019 +86-15383190639 | admin@86-ss.com | China | 1000 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8053 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
Related articles
- L-Proline: Properties, Sources, and Applications
- L-proline is one of the 20 amino acids used by the human body to synthesize proteins. This amino acid is encoded in the human ....
- Aug 16,2024
- A bifunctional catalyst: L-Proline
- L-proline is a natural amino acid having secondary amine functionality and acts as a bifunctional catalyst.
- Nov 14,2023
- Benefits of L-proline
- L-proline plays important roles in protein synthesis and structure, metabolism (particularly the synthesis of arginine, polyam....
- Jan 3,2023
Related Qustion
- Q:What is the function of L-Proline?
- A:L-Proline is the primary amino acid found in cartilage and is important for maintaining youthful skin and repairing muscle, co....
- Dec 4,2023
View Lastest Price from L-Proline manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | L-Proline
147-85-3
|
US $99.00-66.00 / kg | 0.001kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-22 | L-Proline
147-85-3
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd | |
 |
2024-11-22 | L-Proline
147-85-3
|
US $99.00-35.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd |