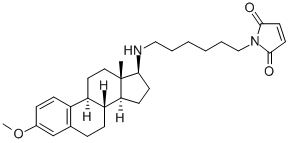
U-73122
- CAS No.
- 112648-68-7
- Chemical Name:
- U-73122
- Synonyms
- U-73312;U-73122;CS-1793;CS-2400;U-73122 98%;U 73122;U-73122;U-73122 hydrate;U-73122 USP/EP/BP;U-73122 hydrate(U73122);U-73122 - CAS 112648-68-7 - Calbiochem
- CBNumber:
- CB6237598
- Molecular Formula:
- C29H40N2O3
- Molecular Weight:
- 464.64
- MDL Number:
- MFCD00893825
- MOL File:
- 112648-68-7.mol
- MSDS File:
- SDS
| Boiling point | 617.1±55.0 °C(Predicted) |
|---|---|
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | ethanol: 0.7 mg/mL |
| form | solid |
| pka | 10.69±0.40(Predicted) |
| color | off-white |
| Water Solubility | Soluble in DMSO, ethanol, or DMF at approx. 0.5mg/ml. May require heating or overnight mixing. Insoluble in water |
| Stability | Stable for 1 year from date of purchase as supplied. Use caution when storing DMSO solutions. Typically solutions in DMSO can be stored at -20°C for approximately 2 months. However, any solutions that turn pink should be discarded. |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
U-73122 price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | U6756 | U-73122 hydrate powder | 112648-68-7 | 5mg | $174 | 2024-03-01 | Buy |
| Sigma-Aldrich | 662035 | U-73122 | 112648-68-7 | 5mg | $172 | 2024-03-01 | Buy |
| Alfa Aesar | J62898 | U-73122, 95% | 112648-68-7 | 1mg | $42.7 | 2023-06-20 | Buy |
| Alfa Aesar | J62898 | U-73122, 95% | 112648-68-7 | 5mg | $154 | 2023-06-20 | Buy |
| Cayman Chemical | 70740 | U-73122 ≥95% | 112648-68-7 | 1mg | $21 | 2024-03-01 | Buy |
U-73122 Chemical Properties,Uses,Production
Description
U-
Uses
It is used as a phospholipase C, phospholipase A2, and 5-LO inhibitor. It is also determined that in SK-N-SH neuroblastoma cells, U-73122 inhibits agonist-induced down-regulation of muscarinic receptors. In addition, it is a useful tool to investigate receptor-mediated PI turnover in signal transduction. U-73122 is a potent inhibitor of human neutrophil adhesion to biological surfaces (IC50 = 50 nM) as well as adhesion-dependent granule exocytosis and oxidative burst. U-73343 (sc-201422) is useful as a negative control for investigations of U-73122 phospholipase C antagonism and its cellular consequences.
Definition
ChEBI: An aza-steroid that is 3-O-methyl-17beta-estradiol in which the 17beta-hydroxy group is replaced by a 6-(maleimid-1-yl)hexylamino group. An inibitor of phospholipase C.
Biological Activity
Phospholipase C inhibitor. Inhibits agonist-induced platelet aggregation with IC 50 values of 1-5 μ M. Potently inhibits human polymorphonuclear neutrophil adhesion on biological surfaces (IC 50 < 50 nM) and exhibits antinociceptive activity in vivo .
Biochem/physiol Actions
Inhibits the hydrolysis of PPI to IP3, which leads to a decrease in cytosolic free calcium. Inhibits the coupling of G protein-phospholipase C activation, while remaining unaffected by production of cAMP.
storage
Store at RT
References
1) Bleasdale?et al. (1990),?Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils; J. Pharmacol. Exp. Ther.,?255?756 2) Zholos?et al.?(2004),?Phospholipase C, but not InsP3 or DAG,-dependent activation of the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells; Br. J. Pharmacol.,?141?23 3) Jun?et al. (2004),?Diacylglycerol and its formation by phospholipase C regulate Rab- and SNARE dependent yeast vacuole fusion; J. Biol. Chem. ,279?53186 4) Fernandez-Ulibarri?et al.?(2007),?Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway; Mol. Biol. Cell,?18?3250
U-73122 Preparation Products And Raw materials
Raw materials
Preparation Products
U-73122 Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22883 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10473 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6391 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38632 | 58 | |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |