Palbociclib
- CAS No.
- 571190-30-2
- Chemical Name:
- Palbociclib
- Synonyms
- Palbociclib API;PD0332991; 571190-30-2;Pabuxilibu;palboclib;bocicL;Pabcilib;Pabuclib;Pab Xilib;Pabsillib
- CBNumber:
- CB62464897
- Molecular Formula:
- C24H29N7O2
- Molecular Weight:
- 447.53
- MDL Number:
- MFCD11840850
- MOL File:
- 571190-30-2.mol
- MSDS File:
- SDS
| Melting point | 200 ºC |
|---|---|
| Boiling point | 711.5±70.0 °C(Predicted) |
| Density | 1.313±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble in DMSO (up to 2 mg/ml with warming) |
| pka | 8.66±0.10(Predicted) |
| form | powder |
| color | white to beige |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| FDA UNII | G9ZF61LE7G |
| NCI Dictionary of Cancer Terms | palbociclib |
| NCI Drug Dictionary | palbociclib |
| ATC code | L01EF01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H341-H361f-H373-H411 |
| Precautionary statements | P201-P202-P260-P273-P280-P308+P313 |
Palbociclib Chemical Properties,Uses,Production
Description
Palbociclib is a cyclin-dependent kinase (CDK) 4 and CDK6 inhibitor approved by the FDA to treat hormone receptor-positive (HR+) human epidural growth factor 2-negative (HER2-) metastatic breast cancer. It is used in combination with letrazole as the first-line hormonal-based therapy in postmenopausal women, or with fulvestrant in women with disease progression following hormonal therapy. Palbociclib was discovered at Warner- Lambert and developed by Pfizer after their merger. Pfizer is also studying the effectiveness of palbociclib in a variety of other cancers at various stages in the clinic.
Uses
Palbociclib (also known as compound number PD-0332991) is an experimental drug for the treatment of breast cancer being developed by Pfizer. It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6.
Indications
Palbociclib (Ibrane(R), Pfizer), a selective CDK4 and CDK6 inhibitor, received accelerated approval from FDA in 2015 for women with estrogen receptor-positive and HER2-negative breast cancer in combination with letrozole.
Definition
ChEBI: A member of the class of pyridopyrimidines that is 2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7-one bearing additional methyl, acetyl and cyclopentyl substituents at positions 5, 6 and 8 respectively. It is used in combina ion with letrozole for the treatment of metastatic breast cancer.
Biochem/physiol Actions
PF-00080665 (Palbociclib; PD 0332991) is an orally active and highly specific inhibitor against cyclin-dependent kinase 4 & 6 (IC50 = 9, 11, 15 nM, respectively, using CDK4/cycD3, CDK4/cycD1, CDK6/cycD2; IC50 >10 μM against 36 other kinases) that potently suppresses Cdk4/6-dependent cellular Rb phosphorylation (IC50 = 66 nM/pSer780 & 63 nM/pSer795; MDA-MB-435). PF-00080665 exhibits selective antiproliferation activity against Rb-positive human breast/colon/lung/leukemia cancer cultures (IC50 = 40-400 nM; IC50 >3 μM/Rb-negative MDA-MB-468 & H2009) and displays in vivo efficacy against various advanced stage human tumor xenografts in mice (12.5-150 mg/kg/day p.o.).
Clinical Use
Protein kinase inhibitor:
Treatment of hormone receptor (HR)-positive,
human epidermal growth factor receptor 2 (HER2)-
negative locally advanced or metastatic breast cancer
Synthesis
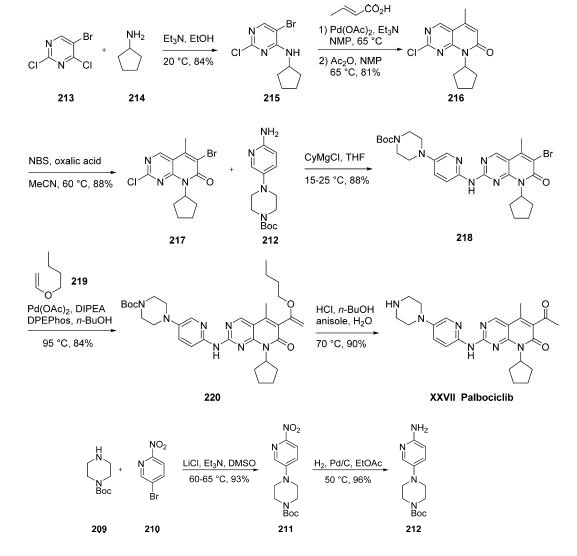
Numerous syntheses of palbociclib have been reported,149,150
and the commercial scale process published by scientists at
Pfizer is described herein. The amino-pyridylpiperazine
fragment 212 was prepared in two steps. Commercial
piperazine 209 was added to 5-bromo-2-nitropyridine (210)
to give nitro-pyridine 211 in 93% yield.
Hydrogenation of the nitro group using catalytic palladium
on carbon provided the amino-pyridylpiperazine 212 in 96%
yield.
As such, cyclopentylamine
(214) was added to 5-bromo-2,4-dichloropyrimidine (213) to
give 5-bromo-2-chloro-6-cyclopentylaminopyrimidine (215) in
84% yield. Heck reaction with crotonic acid followed by
treating the resulting product with acetic anhydride formed the
mixed anhydride under elevated temperatures, and this resulted
in cyclization to give pyrimidinone 214 in 81% yield.
Bromination using N-bromosuccinimide (NBS) provided
coupling partner 217 in 88% yield. Next, aminopyridine 212
was treated with cyclohexylmagnesium chloride and then
reacted with 217 to give the SNAr product 218 in 88%
yield. A second Heck reaction between bromide 218 and
butyl vinyl ether (219) using palladium acetate/bis(2-
diphenylphosphinophenyl)ether (DPEPhos) as the catalyst
provided enol ether 220 in 84% yield. Exposure of 220 to
acidic conditions removed the Boc group from the piperazine
while converting the enol ether to the corresponding ketone,
providing palbociclib (XXVII) in 90% yield.
Enzyme inhibitor
This orally active, non-ATP-competitive cyclin kinase-directed inhibitor (FW = 483.99 g/mol (mono-HCl); CASs = 827022-32-2 (mono- hydrochloride, 571190-30-2 (free base); Solubility: 10 mg/mL DMSO; 30 mg/mL Water; Formulation: Dissolved in sodium lactate buffer (50 mM, ® pH 4.0) ), also known as PD-0332991, Ibrance, and 6-acetyl-8-cyclopentyl- 5-methyl-2- (5- (piperazin-1-yl) pyridin-2-ylamino) pyrido[2,3-d]pyrimidin- 7 (8H) -one hydrochloride, targets Cdk-4 (Cyclin D1) and Cdk-6 (Cyclin D2), enzymes that participate in the so-called CDK4/6-retinoblastoma signaling pathway governing the cell-cycle restriction point. Palbociclib induces rapid G1 cell-cycle arrest in primary human myeloma cells. This agent also shows significant efficacy in a broad spectrum of human tumor xenografts in vivo, resulting in complete regression in some tumors with no evidence of acquired resistance or ability to circumvent the growth inhibitory properties of this agent. Ibrance received FDA approval in 2015 for combined use with letrozole to treat postmenopausal women with estrogen receptor- positive, (HER2) -negative advanced breast cancer as an initial endocrine- based therapy for metastatic disease. Cyclin Target Selectivity: Cdk1 (weak, if any), Cdk2 (weak, if any), Cdk3 (weak, if any), Cdk4 (IC50 = 11 nM), Cdk5 (weak, if any), Cdk6 (IC50 = 16 nM), Cdk7 (weak, if any), Cdk8 (weak, if any), Cdk9 (weak, if any), Cdk10 (weak, if any).
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
clarithromycin - avoid or reduce palbociclib dose;
concentration reduced by rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antifungals: concentration possibly increased
by itraconazole, ketoconazole, posaconazole and
voriconazole - avoid or reduce palbociclib dose.
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid.
Antivirals: concentration possibly increased by
indinavir, lopinavir, ritonavir, saquinavir and
telaprevir - avoid or reduce palbociclib dose.
Cytotoxics: concentration possibly reduced by
enzalutamide - avoid.
Grapefruit juice: concentration possibly increased -
avoid
Metabolism
Palbociclib undergoes extensive hepatic metabolism.
The main metabolic pathways for palbociclib involved
oxidation and sulphonation, with acylation and
glucuronidation contributing as minor pathways.
Unchanged drug accounts for 2.3% and 6.9% of
radioactivity in faeces and urine, respectively. In faeces,
the sulfamic acid conjugate of palbociclib was the major
drug-related component, accounting for 26% of the
administered dose.
References
1) El-Rayes?et al.?(2004),?Cyclooxygenase-2-dependent and –independent effects of celecoxib in pancreatic cancer cell lines; Mol. Cancer Ther.,?3?1427 2) Menu?et al.?(2008),?A novel therapeutic combination using PD 0332991 and bortezomib: study in 5T33MM myeloma model; Cancer Res.,?68?5519 3) Valenzuela?et al.?(2017),?Palbociclib-induced autophagy and senescence in gastric cancer cells; Exp. Cell Res.,?360?390 4) Palanisamy?et al.?(2016),?Palbociclib: A new hope in the treatment of breast cancer; J. Cancer Res. Ther.,?12?1220 5) Goel?et al.?(2017),?CDK4/6 inhibition triggers anti-tumour immunity; Nature?548?471 6)6) AbuHammad?et al.?(2019),?Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma; Proc. Natl. Acad. Sci. USA?116?179909





