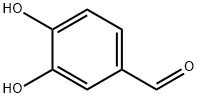
Caffeic acid
- CAS No.
- 331-39-5
- Chemical Name:
- Caffeic acid
- Synonyms
- 3,4-DIHYDROXYCINNAMIC ACID;Caffeic;31-39-5;(E)-3-(3,4-Dihydroxyphenyl)acrylic acid;CAFFIC ACID;3-(3,4-DIHYDROXYPHENYL)ACRYLIC ACID;Caffeic acid 0;4-(2’-carboxyvinyl)-1,2-dihydroxybenzene;β-(3;NSC 623438
- CBNumber:
- CB6281061
- Molecular Formula:
- C9H8O4
- Molecular Weight:
- 180.16
- MDL Number:
- MFCD00004392
- MOL File:
- 331-39-5.mol
- MSDS File:
- SDS
| Melting point | 211-213 °C (dec.) (lit.) |
|---|---|
| Boiling point | 272.96°C (rough estimate) |
| Density | 1.2933 (rough estimate) |
| refractive index | 1.4500 (estimate) |
| storage temp. | 2-8°C |
| solubility | ethanol: 50 mg/mL |
| form | powder |
| pka | 4.58±0.10(Predicted) |
| color | yellow to tan |
| Water Solubility | soluble in hot water |
| Merck | 14,1635 |
| BRN | 2210883 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | QAIPRVGONGVQAS-DUXPYHPUSA-N |
| LogP | 1.150 |
| CAS DataBase Reference | 331-39-5(CAS DataBase Reference) |
| EWG's Food Scores | 3 |
| FDA UNII | U2S3A33KVM |
| Proposition 65 List | Caffeic Acid |
| NCI Drug Dictionary | caffeic acid |
| IARC | 2B (Vol. 56) 1993 |
| NIST Chemistry Reference | Cinnamic acid, 3,4-dihydroxy-(331-39-5) |
| EPA Substance Registry System | 2-Propenoic acid, 3-(3,4-dihydroxyphenyl)- (331-39-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H351 | |||||||||
| Precautionary statements | P280 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 36/37/38-40-63-68 | |||||||||
| Safety Statements | 26-36/37/39-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GD8950000 | |||||||||
| Hazard Note | Irritant | |||||||||
| HS Code | 29182990 | |||||||||
| NFPA 704 |
|
Caffeic acid price More Price(59)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.22029 | 3,4-Dihydroxycinnamic acid for synthesis | 331-39-5 | 10g | $84 | 2024-03-01 | Buy |
| Sigma-Aldrich | 60018 | Caffeic acid matrix substance for MALDI-MS, ≥99.0% (HPLC) | 331-39-5 | 1g | $132 | 2024-03-01 | Buy |
| Sigma-Aldrich | 205546 | Caffeic Acid | 331-39-5 | 500mg | $99.4 | 2024-03-01 | Buy |
| TCI Chemical | C0002 | Caffeic Acid >98.0%(T) | 331-39-5 | 5g | $34 | 2024-03-01 | Buy |
| TCI Chemical | C0002 | Caffeic Acid >98.0%(T) | 331-39-5 | 25g | $106 | 2024-03-01 | Buy |
Caffeic acid Chemical Properties,Uses,Production
Physical and Chemical Properties
Caffeic acid, scientific name: "3-(3,4-dihydroxyphenyl)-2-propenoic acid." Molecular formula: C9H8O4; the molecular weight: 180.16. It is presented in plants such as coffee in the form of chlorogenic acid. It is yellow crystals with melting point being 223~225 °C. When it is precipitated in concentrated solution, it contains no crystal water. However, precipitate of crystals from dilute solution contains one molecule of crystallized water. It is slightly soluble in water, and easily soluble in hot water, cold ethanol, and ethyl acetate. Its alkaline solution is orange and exhibits dark green when being mixed with ferric chloride.
Application: caffeic acid is safe to be applied in cosmetics and has a broader antibacterial and antiviral activity. It can also absorb ultraviolet radiation. A low concentration of it already has inhibitory efficacy on the generation of skin melanin. Its applied amount in the beauty products for whitening is at the range of 0.5 to 2%. It can also be used as additive for the oxidized hair dyes which is good for enhancing the strength of the color.
Preparation: it can be produced from the Perkin’s reaction between protocatechuic aldehyde and acetic acid,As follows:

Extraction Method
Caffeic acid belongs to common phenolic compounds with effects of increasing the levels of white blood cells. It is easily to be confused with caffeine and is widely distributed in the plant kingdom. Its major plant sources include lemon peel, Ranunculaceae cimicifuga rhizome, and valerian root. Caffeic acid, together with ferulic acid, erucic acid, and p-hydroxy cinnamic acid are ubiquitous hydroxy cinnamic acid-class molecule distributed in various kinds of plants. This kind of products has conjugated double bonds in the side chain of the molecular structure, and thus exhibiting significant fluorescence upon ultraviolet light, mostly showing bluish color fluorescence. This is a advantage for paper chromatography tests or thin layer chromatography tests.
Extraction Method: Single spike cimicifuga rhizome is extracted with methanol which is removed through concentration under reduced pressure. Add hot water to the residue to dissolve it. Heating the water to dissolve the residue, and further filter the insolubles upon heating. After cooling, add benzene for extraction with the benzene solution being washed with 1% aqueous sodium bicarbonate and further collecting the washed solution. Add dilute hydrochloric acid for acidification, and then apply benzene to remove the free organic acid; Concentrate under reduced pressure to get rid of the benzene with the residue being the enriched product of caffeic acid.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Drugs for white blood cells increase
Caffeic acid is a kind of drugs for stopping bleeding as well as increasing the number of white blood cell with effects of contracting microvascular coagulation, improving the function of coagulation factors, and increasing white blood cells and platelets. It is suitable for preventing bleeding or stopping bleeding in surgeries as well as for stopping bleeding for bleeding diseases in internal medicine, and obstetrics and gynecology. It is also suitable for treating leukopenia and thrombocytopenia caused by a variety of reasons.
HPLC determination of caffeic acid in dandelion
[For the test] Compositae dandelion: Taraxacum mongolicum Hand. Mazz, Alkali land dandelion T. sinicum Kitag.
(1) Chromatographic conditions: take octadecylsilane silica gel as a filling agent; methanol-phosphate buffer (sodium dihydrogen phosphate 1.56g, add water to dissolve it in 1000 ml, add 1% of phosphoric acid solution for adjusting to pH 3.8 to 4.0, that’s it) (23:77) as the mobile phase; detection wavelength is 323 nm; column temperature should be 40 °C. Number of theoretical plates should be calculated according to the caffeic acid peak and should not be less than 3,000.
(2) the preparation of the reference solution: take 7.5 mg of caffeic acid reference substance, accurately weigh it and transfer it into the 50ml volumetric flask; add methanol to certain scale, shake; take precise amount of 2ml and put into 10ml volumetric flask, add methanol to the scale , shake, to obtain the reference solution (containing caffeic acid 30μg per ml).
(3) Preparation of sample solution: Take about 1 g of medicine powder, accurately weigh it, and put it in 50ml Erlenmeyer flask, precisely add 10 mL of methanol solution containing 5% formic acid, seal, shake, weigh, and subject to ultrasonic treatment for 30min, remove, cool, then weigh again; use the methanol solution of 5% formic acid to complement the weight loss, shake, centrifuge, and take the supernatant for being filtrated through microporous membrane (0.45μm) with the filtrate being placed in brown bottle to obtain the sample solution.
(4) Determination: separately and precisely pipette 10μl of both reference solution and sample solution and transfer into the liquid chromatography for measurement.
(5) Measurement results: calculated from the dry products of dandelion herbs, the caffeic acid content should not be less than 0.02%.
Chemical Properties
It is yellow crystals and can be dissolved in water and ethanol.
Uses
1. Reagents for Organic Synthesis. 2. Intermediate of caffeic acid; can be used in organic synthesis. 3. Used for Biochemical studies.
Category
Toxic Chemicals
Toxicity grading
Poisoning
Acute toxicity
Intraperitoneal administration-rat LDL0: 1500 mg/kg
Flammability and hazard characteristics
Thermal decomposition causes irritating smoke
Storage Characteristics
Treasury: ventilation low-temperature and dry.
Extinguishing agent
Water, powder, CO2, foam.
Description
Caffeic acid is abundant in the whole plant of Solidago decurrens Lour. (Yi Zhi
Huang Hua), fruit of Crataegus pinnatifida Bge. var. major N.E.Br. (Shan Li
Hong), Salix myrtillacea Anderss. (Po Liu), rhizome of Cimicifuga foetida L., rhizome of Polypodiaceae Polypodium vulgare L. (Ou Ya Shui Long Gu), peel of
Rutaceae Citrus limonum (Ning Meng), the whole plant of Polygonaceae Polygonum
aviculare L. (Pian Xu), root of Valeriana officinalis L. (Xie Cao), the whole plant of
Thymus mongolicus Ronn (She Xiang), leaves of Eucommia ulmoides (Du Zhong),
and other herbal plants. It is a kind of polyhydroxy styrene acid, with the general
chemical properties of phenolic acid. It is easily oxidized for the reason of the unsaturated double bonds, particularly unstable in alkaline solution
Caffeic acid has both cis and trans isomers, and the two isomers of caffeic acid
have a mutual transformation in plants, which may regulate some important physiological process. Caffeic acid exists in plants in the main form of complexes; free
state accounts for a few proportion.
Description
Caffeic acid is an inhibitor of 5-
Chemical Properties
Light yellow to greenish-yellow powder
Physical properties
Appearance: yellow crystal. The crystal from the concentrated solution does not
contain crystal water, and the crystal from the dilute solution contains one molecule
crystal water. Melting point: 223?225?°C. Solubility: It is slightly soluble in cold
water but soluble in hot water, cold ethanol, and ethyl acetate. The basic solution is
orange-red. Ferric chloride solution was dark green.
Uses
antineoplastic, PGE2 synthase inhibitor, PK inhibitor
Uses
Caffeic acid has been used as a standard of phenolic acid in the study to determine the total phenolic acid content in vegetables after subjecting to alkaline and acid hydrolysis. It has also been used to determine its antioxidant activity by various assay methods.
Uses
Caffeic Acid is a constituent of plants, probably occurs in plants only in conjugated forms. Caffeic acid is found in all plants because it is a key intermediate in the biosynthesis of lignin, one of the principal sources of biomass. Caffeic acid is one of the main natural phenols in argan oi.
Definition
ChEBI: Caffeic acid is a hydroxycinnamic acid that is cinnamic acid in which the phenyl ring is substituted by hydroxy groups at positions 3 and 4. It exists in cis and trans forms; the latter is the more common. It has a role as a plant metabolite, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, an EC 2.5.1.18 (glutathione transferase) inhibitor, an EC 1.13.11.34 (arachidonate 5-lipoxygenase) inhibitor, an antioxidant and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is a hydroxycinnamic acid and a member of catechols.
Indications
It is used for preventing or stopping bleeding during surgery, as well as hemostasis in the department of medicine, obstetrics and gynecology, etc. It is also used for various causes of neutropenia and thrombocytopenia.
General Description
Yellow prisms or plates (from chloroform or ligroin) or pale yellow granules. Alkaline solutions turn from yellow to orange.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Caffeic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Caffeic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Caffeic acid emits acrid smoke and fumes.
Fire Hazard
Flash point data for Caffeic acid are not available; however, Caffeic acid is probably combustible.
Biochem/physiol Actions
A natural dietary phenolic compound found in plants that is an anti-oxidant. Inhibits the synthesis of leukotrienes that are involved in immunoregulation, inflammation and allergy. Inhibits Cu2+-induced LDL oxidation.
Purification Methods
Recrystallise this antioxidant from water. [Beilstein 10 IV 1776.]
Caffeic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Rochi Pharmaceutical Co.,Ltd. | 21-38751876 +8615000076078 | info@rochipharma.com | China | 431 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Junwee Chemical co., Ltd. | +86-571-85808636 +86-13958122542 | sales@junweechem.com | China | 821 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 376 | 58 |
| SUZHOU NMT BIOTECH CO.,LTD | +86-0512-62388427 +86-17306196472 | nmtdianzi@163.com | China | 41 | 58 |
| Watson Biotechnology Co.,Ltd | +86-18186686046 +86-18186686046 | sales01@watsonbiotech.cn | China | 5822 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2964 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +8618580541567 | sales@zhswyy.com | China | 303 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
Related articles
- In what foods and plants does Caffeic acid occur?
- Caffeic acid occurs in almost all plants. Health Benefits Consumption of CA-rich foods leads to a protective effect against c....
- Nov 23,2023
- Caffeic acid: pharmacokinetics and clinical applications
- Caffeic acid, a natural polyphenol, has health benefits including antioxidant effects, potential cancer prevention, diabetes m....
- Jul 24,2023
- Caffeic acid - Health Benefits
- Caffeic acid is a polyphenol, a class of micronutrients found in plant foods. More specifically, caffeic acid is part of a gro....
- Jan 12,2022
Related Qustion
- Q:What are the Biological activity and Toxicity of caffeic acid
- A:Caffeic acid is an orally bioavailable, hydroxycinnamic acid derivative and a polyphenol, with antioxidant, anti-inflammatory,....
- Sep 14,2024
View Lastest Price from Caffeic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Caffeic acid
331-39-5
|
US $200.00-85.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-22 | Caffeic acid
331-39-5
|
US $0.00-0.00 / kg | 1kg | 99% | 20MT | Watson Biotechnology Co.,Ltd | |
 |
2024-11-22 | Caffeic acid
331-39-5
|
US $0.00-0.00 / kg | 1kg | ≥98% HPLC | 10000 | Changsha Staherb Natural Ingredients Co., Ltd. |
-

- Caffeic acid
331-39-5
- US $200.00-85.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Caffeic acid
331-39-5
- US $0.00-0.00 / kg
- 99%
- Watson Biotechnology Co.,Ltd
-

- Caffeic acid
331-39-5
- US $0.00-0.00 / kg
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.