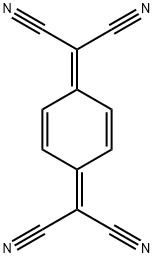
7,7,8,8-Tetracyanoquinodimethane
- CAS No.
- 1518-16-7
- Chemical Name:
- 7,7,8,8-Tetracyanoquinodimethane
- Synonyms
- TCNQ;TETRACYANOQUINODIMETHANE;Tetracyanoquinodimethan;Tetracyano p-Quinone Dimethane;TIMTEC-BB SBB000435;7,7,8,8-Tetracyanoqu;tetracyano-quinodimetha;etracyanoquinodimethane;7,7,8,8-Tetracyanodimethan;Quinodimethan, tetracyano-
- CBNumber:
- CB6306980
- Molecular Formula:
- C12H4N4
- Molecular Weight:
- 204.19
- MDL Number:
- MFCD00011664
- MOL File:
- 1518-16-7.mol
- MSDS File:
- SDS
| Melting point | 287-289 °C (dec.) (lit.) |
|---|---|
| Boiling point | 332.67°C (rough estimate) |
| Density | 1.3596 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| form | Crystalline Powder |
| color | Orange-brown to green |
| Water Solubility | insoluble |
| λmax | 395nm(CH3CN)(lit.) |
| BRN | 611604 |
| Stability | Stable. Incompatible with strong acids, strong bases, strong reducing agents, strong oxidizing agents. |
| InChI | InChI=1S/C12H4N4/c13-5-11(6-14)9-1-2-10(4-3-9)12(7-15)8-16/h1-4H |
| InChIKey | PCCVSPMFGIFTHU-UHFFFAOYSA-N |
| SMILES | C1(=C(C#N)C#N)C=CC(=C(C#N)C#N)C=C1 |c:8,t:0| |
| CAS DataBase Reference | 1518-16-7(CAS DataBase Reference) |
| FDA UNII | HC6FB4H2KW |
| NIST Chemistry Reference | Propanedinitrile, 2,2'-(2,5-cyclohexadiene-1,4-diylidene)bis-(1518-16-7) |
| EPA Substance Registry System | Propanedinitrile, 2,2'-(2,5-cyclohexadiene-1,4-diylidene)bis- (1518-16-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331 | |||||||||
| Precautionary statements | P280-P301+P310+P330-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | Xn,Xi,T | |||||||||
| Risk Statements | 22-20/21/22-23/24/25 | |||||||||
| Safety Statements | 22-24/25-36/37-26-36-45 | |||||||||
| RIDADR | 3276 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GU4850000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29269095 | |||||||||
| NFPA 704 |
|
7,7,8,8-Tetracyanoquinodimethane price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 157635 | 7,7,8,8-Tetracyanoquinodimethane 98% | 1518-16-7 | 5g | $108 | 2024-03-01 | Buy |
| Sigma-Aldrich | 157635 | 7,7,8,8-Tetracyanoquinodimethane 98% | 1518-16-7 | 10g | $155 | 2024-03-01 | Buy |
| TCI Chemical | T0078 | 7,7,8,8-Tetracyanoquinodimethane >98.0%(HPLC)(N) | 1518-16-7 | 5g | $63 | 2024-03-01 | Buy |
| TCI Chemical | T0078 | 7,7,8,8-Tetracyanoquinodimethane >98.0%(HPLC)(N) | 1518-16-7 | 25g | $215 | 2024-03-01 | Buy |
| Alfa Aesar | A10779 | 7,7,8,8-Tetracyanoquinodimethane, 98% | 1518-16-7 | 5g | $115 | 2024-03-01 | Buy |
7,7,8,8-Tetracyanoquinodimethane Chemical Properties,Uses,Production
Applications
7,7,8,8-tetracyanoquinodimethane (TCNQ), with a LUMO at 4.5 eV, is known for the charge-transfer salts formed by its radical anion TCNQ in photovoltaic, light-emitting diodes, and organic field-effect transistor devices. TCNQ and its derivatives have been used as dopants, leading to an increase in hole mobility or to the lowering of injection barriers. One classic example of such is the treatment of tetrathiafulvene (TTF), an electron donor with TCNQ. TFF and TCNQ form an ion pair, the TTF-TCNQ complex. This process of doping leads to the crystallisation of the ion pair into a one-dimensionally stacked polymer. This polymer consists of segregated stacks of cations and anions of the donors and the acceptors, respectively. The complex crystal is an organic semiconductor that exhibits metallic electric conductivity.
It also has been shown that TCNQ can effectively modify Cu or Ag surfaces. The formation of Cu-TCNQ and Ag-TCNQ enhances the work function of such electrodes and reduces the hole injection barrier dramatically. Furthermore, it improves electrode/organic layer contact, hence the reduction of contact resistances.
Tetracyanoquinodimethane (TCNQ) is also found to act as a p-type doping agent of graphene films due to its powerful electron-accepting capacity.
Description
7,7,8,8-tetracyanoquinodimethane (TCNQ), with a LUMO at 4.5 eV, is known for the charge-transfer salts formed by its radical anion TCNQ in?photovoltaic, light-emitting diodes, and organic field-effect transistor?devices. TCNQ and its derivatives?have been used as dopants, leading to an increase in hole mobility or to the lowering of injection?barriers. One classic example of such is the treatment of tetrathiafulvene (TTF), an electron donor with TCNQ. TFF and TCNQ form an ion pair, the TTF-TCNQ complex. This process of doping leads to the crystallisation of the ion pair into a one-dimensionally stacked polymer. This polymer consists of segregated stacks of cations and anions of the donors and the acceptors, respectively. The complex crystal is an organic semiconductor that exhibits metallic electric conductivity [1, 2].
Chemical Properties
orange to green crystalline powder
History
The first report on the electrical conductivity in an organic solid appeared in 1954, namely, a perylene—bromine complex with a room-temperature conductivity of 0.1 S cm?1. In 1960, the organic acceptor Tetracyanoquinodimethane (TCNQ; 7,7,8,8-Tetracyanoquinodimethane) was synthesized, as well as a great number of its conducting charge-transfer complexes and radical ion salts. In the 1970s, the organic donor TTF led to the first organic metal TTF-TCNQ. Its room-temperature conductivity (500 S cm?1) increases with a decrease in the temperature to the value of 6000 S cm?1 at 60 K, where a metal-insulator transition occurs[1].
Uses
7,7,8,8-Tetracyanoquinodimethane is an electron-acceptor molecule used to form charge-transfer superconductors. It is an effective catalyst used for the ?-chlorination of carboxylic acids using chlorosulfonic acid; the presence of TCNQ suppresses competing free-radical chlorination.
Definition
ChEBI: A quinodimethane that is p-quinodimethane in which the methylidene hydrogens are replaced by cyano groups.
Synthesis Reference(s)
Journal of the American Chemical Society, 84, p. 3370, 1962 DOI: 10.1021/ja00876a028
The Journal of Organic Chemistry, 48, p. 1366, 1983 DOI: 10.1021/jo00156a048
General Description
7,7,8,8-Tetracyanoquinodimethane (TNCQ) is a strong electron acceptor as it has four cyano groups and π-conjugation bonds that form charge transferring chains and ion radical salts which are mainly used as p-dopants for the fabrication of a variety of semiconductor applications.
References
[1] Tahar Abbaz. “Synthesis and electrochemical proprieties of novel unsymmetrical bis-tetrathiafulvalenes and electrical conductivity of their charge transfer complexes with tetracyanoquinodimethane (TCNQ).” International Journal of Molecular Sciences 13 7 (2012): 7872–7885.
7,7,8,8-Tetracyanoquinodimethane Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Lidu New Material Technology Co., Ltd | +undefined+86-19307248857 | liduxcl@163.com | China | 1134 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10986 | 58 |
| Speranza Chemical Co., Ltd. | +86-86-075521030354 +8618688942810 | sophieliu@speranzachem.com | China | 802 | 55 |
| Jilin Chinese Academy of Sciences - Yanshen Technology Co., Ltd. | 0431-80514535 13634302652 | Extension@chemextension.com | CHINA | 967 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29811 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5600 | 58 |
| Kindchem(Nanjing)Co.,Ltd | +86-025-025-85281586 +8618651653755 | sales@kindchem.cn | China | 1226 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34553 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9639 | 58 |
View Lastest Price from 7,7,8,8-Tetracyanoquinodimethane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | 7,7,8,8-TETRACYANOQUINODIMETHANE
1518-16-7
|
US $0.00-0.00 / g | 10g | 98% | 20tons | Hubei Lidu New Material Technology Co., Ltd | |
 |
2024-08-27 | 7,7,8,8-Tetracyanoquinodimethane
1518-16-7
|
US $0.10-0.30 / kg | 1kg | 98% | 20 tons | Zibo Hangyu Biotechnology Development Co., Ltd | |
 |
2020-01-09 | 7,7,8,8-Tetracyanoquinodimethane
1518-16-7
|
US $1.00 / KG | 1KG | 99% | 100KG | Career Henan Chemical Co |
-

- 7,7,8,8-TETRACYANOQUINODIMETHANE
1518-16-7
- US $0.00-0.00 / g
- 98%
- Hubei Lidu New Material Technology Co., Ltd
-

- 7,7,8,8-Tetracyanoquinodimethane
1518-16-7
- US $0.10-0.30 / kg
- 98%
- Zibo Hangyu Biotechnology Development Co., Ltd
-

- 7,7,8,8-Tetracyanoquinodimethane
1518-16-7
- US $1.00 / KG
- 99%
- Career Henan Chemical Co
1518-16-7(7,7,8,8-Tetracyanoquinodimethane)Related Search:
1of4