7,7,8,8-テトラシアノキノジメタン 化学特性,用途語,生産方法
外観
うすい黄色~くすんだ黄色~暗い緑色粉末~結晶
解説
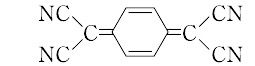
2,2′-(2,5-cyclohexadiene-1,4-diylidene)bispropanedinitrile.C12H4N4(204.19).略称TCNQ.シクロヘキサン-1,4-ジオンとマロノニトリルとを縮合させて1,4-ビス(ジシアノメチレン)シクロヘキサンとし,ついで臭素化,脱臭化水素して合成する.
"橙色の結晶.分解点297~298 ℃.アセトニトリル,ジオキサン,THFなどに可溶.強力な電子受容体として分子錯体を形成するほか,多数の単純および複雑陰イオンラジカル塩,M+ (TCNQ)n(n = 1,4/3,3/2,2)などを与え,高電導性のものも多い.電導性および光電導性複合材料として,ケミカルコンデンサーなどの電子材料,電子写真用感光材料に用いられる.[CAS 1518-16-7]
森北出版「化学辞典(第2版)
説明
7,7,8,8-tetracyanoquinodimethane (TCNQ), with a LUMO at 4.5 eV, is known for the charge-transfer salts formed by its radical anion TCNQ in?photovoltaic, light-emitting diodes, and organic field-effect transistor?devices. TCNQ and its derivatives?have been used as dopants, leading to an increase in hole mobility or to the lowering of injection?barriers. One classic example of such is the treatment of tetrathiafulvene (TTF), an electron donor with TCNQ. TFF and TCNQ form an ion pair, the TTF-TCNQ complex. This process of doping leads to the crystallisation of the ion pair into a one-dimensionally stacked polymer. This polymer consists of segregated stacks of cations and anions of the donors and the acceptors, respectively. The complex crystal is an organic semiconductor that exhibits metallic electric conductivity [1, 2].
化学的特性
orange to green crystalline powder
来歴
The first report on the electrical conductivity in an organic solid appeared in 1954, namely, a perylene—bromine complex with a room-temperature conductivity of 0.1 S cm?1. In 1960, the organic acceptor Tetracyanoquinodimethane (TCNQ; 7,7,8,8-Tetracyanoquinodimethane) was synthesized, as well as a great number of its conducting charge-transfer complexes and radical ion salts. In the 1970s, the organic donor TTF led to the first organic metal TTF-TCNQ. Its room-temperature conductivity (500 S cm?1) increases with a decrease in the temperature to the value of 6000 S cm?1 at 60 K, where a metal-insulator transition occurs[1].
使用
7,7,8,8-Tetracyanoquinodimethane is an electron-acceptor molecule used to form charge-transfer superconductors. It is an effective catalyst used for the ?-chlorination of carboxylic acids using chlorosulfonic acid; the presence of TCNQ suppresses competing free-radical chlorination.
定義
ChEBI: A quinodimethane that is p-quinodimethane in which the methylidene hydrogens are replaced by cyano groups.
一般的な説明
7,7,8,8-Tetracyanoquinodimethane (TNCQ) is a strong electron acceptor as it has four cyano groups and π-conjugation bonds that form charge transferring chains and ion radical salts which are mainly used as p-dopants for the fabrication of a variety of semiconductor applications.
7,7,8,8-テトラシアノキノジメタン 上流と下流の製品情報
原材料
準備製品