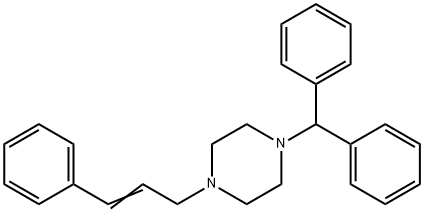
Stugeron
- CAS No.
- 298-57-7
- Chemical Name:
- Stugeron
- Synonyms
- CINNARIZINE;Cinarizine;Cinnarizine, powder;Cinnarizine Hydrochloride;r516;R 516;r1575;Sepan;516md;Eglen
- CBNumber:
- CB6384748
- Molecular Formula:
- C26H28N2
- Molecular Weight:
- 368.51
- MDL Number:
- MFCD00056037
- MOL File:
- 298-57-7.mol
- MSDS File:
- SDS
| Melting point | 117-120°C |
|---|---|
| Boiling point | 488.83°C (rough estimate) |
| Density | 1.1818 (rough estimate) |
| refractive index | 1.7620 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Soluble in chloroform at 50mg/ml |
| pka | pKa 7.47(H2O t=25.0 ) (Uncertain) |
| form | powder |
| color | white |
| Water Solubility | 0.75g/L(temperature not stated) |
| Merck | 14,2305 |
| InChIKey | DERZBLKQOCDDDZ-JLHYYAGUSA-N |
| CAS DataBase Reference | 298-57-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 3DI2E1X18L |
| NIST Chemistry Reference | Cinnarizine(298-57-7) |
| ATC code | N07CA02,N07CA52 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H360-H410 |
| Precautionary statements | P273-P391-P501-P264-P280-P302+P352-P321-P332+P313-P362 |
| Hazard Codes | Xn |
| Risk Statements | 20/22-36/37/38-42/43 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 2 |
| RTECS | TL3430000 |
| HS Code | 2933.59.2100 |
| Toxicity | LD50 oral in rat: > 6500mg/kg |
Stugeron price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR2811 | Cinnarizine certified reference material, pharmaceutical secondary standard | 298-57-7 | 200MG | $365 | 2024-03-01 | Buy |
| Sigma-Aldrich | C5270 | Cinnarizine powder | 298-57-7 | 10g | $57 | 2024-03-01 | Buy |
| Sigma-Aldrich | C2180000 | Cinnarizine European Pharmacopoeia (EP) Reference Standard | 298-57-7 | c2180000 | $153 | 2024-03-01 | Buy |
| TCI Chemical | C3459 | Cinnarizine >98.0%(HPLC)(N) | 298-57-7 | 10g | $52 | 2024-03-01 | Buy |
| TCI Chemical | C3459 | Cinnarizine >98.0%(HPLC)(N) | 298-57-7 | 50g | $149 | 2024-03-01 | Buy |
Stugeron Chemical Properties,Uses,Production
Peripheral vasodilator
Stugeron is a piperazine calcium channel blockers, belonging to the peripheral vasodilator. It is white or white-like crystal or crystalline powder. It is odorless and almost tasteless. It is insoluble in water, soluble in boiling ethanol and easily soluble in chloroform or benzene. Stugeron has dilatation effect on the vascular smooth muscle and has antagonism effect on various kinds of vasoconstrictor substances such as serotonin, epinephrine, bradykinin and vasopressin, etc., it can ease the vasospasm. Its antispasmodic effect is stronger than papaverine with a not obvious sedative effect. This product can enables a significant increase in the cerebral blood flow and can significantly improve the cerebral circulation and coronary circulation with longer duration of action and has inhibitory effect on various kinds of vasoactive substances. It is capable of relieving vasospasm and preventing the embrittlement of the blood vessel. In the situation of not affecting heart rate and oxygen consumption, it can increase the coronary blood flow and cardiac output. Intravenous injection of this product can cause short period of blood pressure drop while oral administration has no such effect. In addition, this product also has effect on preventing embrittlement of the blood vessel as well as anti-histamine effect.
It will onset within 30 minutes after oral administration with the plasma concentration reaching peak within 3-7 hours. The effect can last for four hours with the half-life of plasma concentration being 3 to 24 hours.
Clinically Stugeron is mainly used for the treatment of peripheral vascular disease and cerebrovascular disorders such as cerebral embolism, cerebral thrombosis, cerebral arteriosclerosis and hypertension induced circulation insufficiency, cerebral hemorrhage, subarachnoid hemorrhage convalescence and sequelae of traumatic brain injury. It also has efficacy in treating the mental neuropathy and coronary sclerosis. It can also be used for treating the inner ear vertigo and nausea, vomiting and motion sickness caused by other disorders.
The main adverse reactions of Stugeron: occasionally drowsiness, rash, gastrointestinal reactions, dizziness, fever or flu, intravenous blood pressure can cause short-term decline of the blood pressure; pregnant women should take with caution; patients of intracranial hemorrhage and acute cerebral infarction should be hanged.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Chemical Properties
Stugeron hydrochloride, C26H28N2 • 2HCI, [7002-58-6], melting point 192 ℃, the solubility in water is 2mg/100ml.
Uses
It is long-efficacy, multifunction vasoconstriction antagonists. It can dilate blood vessels, improve blood circulation and prevent blood vessel embrittlement. It can be used in the treatment of cerebral vascular diseases as well as has efficacy in treating cervical vertigo caused headaches, dizziness, insomnia, memory loss, paralysis, numbness, weakness and slurred speech embolism.
Production method
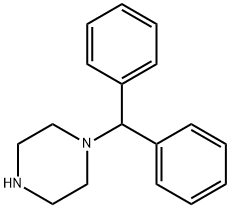
It can be produced through: first use anhydrous piperazine and brominated diphenyl methane for preparation of benzhydryl piperazine, then condense with chlorine allyl benzene to obtain it. Diphenyl methane was heated in the light, add bromine and incubate for 1h at 130 ℃ which generates brominated diphenyl methane, C13H11Br, [776-74-9] with the melting point of 45 ℃. Add the brominated diphenyl methane drop wise into the piperazine toluene and stir for 3h at 80-90 ℃, further wash with water after cooling, then use 10% dilute hydrochloric acid for extraction; The acid layer was basified for being precipitated, filtered and dried to give benzhydryl piperazine. Dissolve it in 95% ethanol, add sodium carbonate and add drop wise of allyl benzene at about 65 ℃, after the completion of the dropping, heat and reflux for 4h, filter hot and the filtrate was left overnight for precipitate the crystalline, filter to get the crude product of Stugeron which under refinement become finished product with melting point of 117-119 ℃. In terms of diphenyl methane, the total yield is 48.2%.
Chemical Properties
White or almost white powder.
Originator
Stugeron,Janssen,UK,1961
Uses
For the treatment of vertigo/meniere's disease, nausea and vomiting, motion sickness and also useful for vestibular symptoms of other origins.
Uses
Histamine H1 receptor antagonist; antihistamine.
Uses
glucocorticoid, antiinflammatory
Uses
Calcium channel blocker with antiallergic and anti-vasoconstricting activity. Antihistaminic
Definition
ChEBI: Cinnarizine is a N-alkylpiperazine, a diarylmethane and an olefinic compound. It has a role as an antiemetic, a histamine antagonist, a calcium channel blocker, a muscarinic antagonist, an anti-allergic agent, a H1-receptor antagonist and a geroprotector.
Manufacturing Process
This compound can be prepared by the reaction of cinnamoyl chloride with
benzhydryl piperazine. The reaction is carried out in dry benzene under reflux.
The benzene is then evaporated, the residue taken up in chloroform, washed
with dilute HCl and then made alkaline.
The chloroform layer is washed with a dilute aqueous sodium hydroxide
solution, thereafter with water, and is finally dried over potassium carbonate.
The residue, which is obtained after evaporation of the chloroform, is
dissolved by heating in a mixture of 25% of toluene and 75% of heptane. On
cooling this solution to about 20°C the product precipitates. That compound is
reduced with LiAlH4, to give cinnarizine.
brand name
Rinomar.
Therapeutic Function
Antihistaminic
World Health Organization (WHO)
Cinnarizine, an antihistaminic and vasodilator agent, was introduced into medicine in 1962. It is indicated for the treatment of labyrinthine disturbances and vascular disorders, although its effectiveness in the latter indication has not been convincingly demonstrated.
General Description
Cinnarizine is a piperazine derivative, which is extracted from wood reed roots. It exhibits antihistaminic and calcium antagonist property. Cinnarizine is used to treat vertigo, unsteadiness and cognitive disorders. Cinnarizine has anticholinergic, antiserotonergic and antidopaminergic effects. It enhances cerebral blood flow. Cinnarizine blocks the contraction of smooth muscles cells and also acts as a skin whitening agent.
Biological Activity
cinnarizine is a calcium channel blocker.blockers of calcium channel are drugs disrupting the calcium movement via calcium channels, which are usually used as antihypertensive therapies to decrease blood pressure in hypertension patients.
Biochem/physiol Actions
Cinnarizine is a piperazine and a specific anti-vertigo agent. It is used to treat and prevent vertigo and motion sickness. In addition, cinnarizine is also used as an anti-histamine agent. Chronic use of this drug leads to side effects such as extrapyramidal reactions (Parkinson, tremor and akathisia) and depression.
Mechanism of action
In addition to its antihistaminic activity, cinnarizine possesses Ca2+ channel blocking activity. Its vasodilator effect probably results from blocking the Ca2+ channels in the vascular smooth muscle cells.
Clinical Use
Vestibular disorders
Motion sickness
Synthesis
Cinnarinzine is prepared by alkylation of 1-diphenylmethyl piperazine with 3-phenyl-2-propenyl chloride .
in vitro
previous study found that cinnarizine could inhibit the phosphorylation of both arterial myosin p-light chain and arterial actomyosin superprecipitation. moreover, the concomitant inhibition of arterial superprecipitation and phosphorylation by perhexiline and cinnarizine was found to be similar to that of w-7. such inhibitary effect was then characterized by a rightward shift in the pca superprecipitation, depressed maximum activity as well as attenuation by exogenous calmodulin [1].
in vivo
previous animal study showed that augmented effects were obtained in mes seizure model when cinnarizine was combined with sodium valproate. whereas, in ptz-induced seizures, augmented effects were obtained when nifedipine was combined with sodium valproate [2].
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: possibly increased sedative effects with
opioid analgesics.
Metabolism
Cinnarizine is extensively metabolised mainly via CYP2D6, but there is considerable inter-individual variation in the extent of metabolism. The elimination of metabolites occurs as follows: one third in the urine (unchanged as metabolites and glucuronide conjugates) and two thirds in the faeces.
References
[1] silver pj,dachiw j,ambrose jm,pinto pb. effects of the calcium antagonists perhexiline and cinnarizine on vascular and cardiac contractile protein function. j pharmacol exp ther.1985 sep;234(3):629-35.
[2] brahmane ri,wanmali vv,pathak ss,salwe kj. role of cinnarizine and nifedipine on anticonvulsant effect of sodium valproate and carbamazepine in maximal electroshock and pentylenetetrazole model of seizures in mice. j pharmacol pharmacother.2010 jul;1(2):78-81.
[3] hausler r,sabani e,rohr m. effect of cinnarizine on various types of vertigo. clinical and electronystagmographic results of a double-blind study. acta otorhinolaryngol belg.1989;43(2):177-85.
Stugeron Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
View Lastest Price from Stugeron manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Stugeron
298-57-7
|
US $0.00 / Kg/Drum | 25KG | 99.0%~101.0% | 10tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Cinnarizine
298-57-7
|
US $50.00 / mL | 100% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Cinnarizine
298-57-7
|
US $50.00 / mL | 100% | 10g | TargetMol Chemicals Inc. |
-

- Stugeron
298-57-7
- US $0.00 / Kg/Drum
- 99.0%~101.0%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Cinnarizine
298-57-7
- US $50.00 / mL
- 100%
- TargetMol Chemicals Inc.
-

- Cinnarizine
298-57-7
- US $50.00 / mL
- 100%
- TargetMol Chemicals Inc.