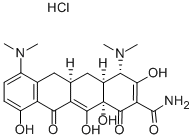
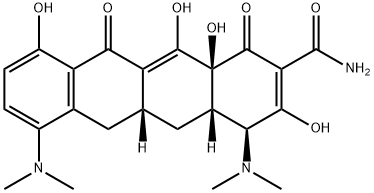
Minocycline hydrochloride
- CAS No.
- 13614-98-7
- Chemical Name:
- Minocycline hydrochloride
- Synonyms
- MINOCYCLINE HCL;Arestin;Dynacin;minocyclinechloride;[4S-(4alpha,4aalpha,5aalpha,12aalpha)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxonaphthacene-2-carboxamide monohydrochloride;minocyn;MINOCIN;tri-mino;MYNOCINE;Minomycin
- CBNumber:
- CB6472139
- Molecular Formula:
- C23H28ClN3O7
- Molecular Weight:
- 493.94
- MDL Number:
- MFCD00083669
- MOL File:
- 13614-98-7.mol
- MSDS File:
- SDS
| Melting point | 205-210° (dec) |
|---|---|
| Boiling point | 813℃ |
| Flash point | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble in water, slightly soluble in ethanol (96 per cent). It dissolves in solutions of alkali hydroxides and carbonates. |
| form | crystalline |
| color | yellow |
| Water Solubility | Freely soluble in water |
| Merck | 14,6202 |
| Stability | Light Sensitive |
| InChIKey | GLMUAFMGXXHGLU-VQAITOIOSA-N |
| SMILES | [C@@]12([H])C[C@@]3([H])C(C(=O)C4C(O)=CC=C(N(C)C)C=4C3)=C(O)[C@]1(O)C(=O)C(C(=O)N)=C(O)[C@H]2N(C)C.Cl |&1:0,3,21,31,r| |
| LogP | 0.808 (est) |
| CAS DataBase Reference | 13614-98-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 0020414E5U |
| Proposition 65 List | Minocycline Hydrochloride (internal use) |
| NCI Drug Dictionary | Dynacin |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H317-H360-H362-H303-H315-H319-H335 | |||||||||
| Precautionary statements | P201-P202-P260-P263-P264-P270-P272-P280-P302+P352+P333+P313+P363-P308+P313-P405-P501-P261-P280a-P304+P340-P305+P351+P338-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QI7630500 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29413020 | |||||||||
| Toxicity | human,TDLo,oral,14286ug/kg/10 (14.286mg/kg),LUNGS, THORAX, OR RESPIRATION: RESPIRATORY OBSTRUCTIONLUNGS, THORAX, OR RESPIRATION: DYSPNEASKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE",Archives of Internal Medicine. Vol. 154, Pg. 1633, 1994. | |||||||||
| NFPA 704 |
|
Minocycline hydrochloride price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1444004 | Minocycline hydrochloride United States Pharmacopeia (USP) Reference Standard | 13614-98-7 | 350mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | M2288 | Minocycline Hydrochloride >98.0%(HPLC)(T) | 13614-98-7 | 1g | $171 | 2024-03-01 | Buy |
| TCI Chemical | M2288 | Minocycline Hydrochloride >98.0%(HPLC)(T) | 13614-98-7 | 5g | $556 | 2024-03-01 | Buy |
| Alfa Aesar | J66429 | Minocycline hydrochloride, 890-950 μg/mg | 13614-98-7 | 500mg | $234 | 2024-03-01 | Buy |
| Alfa Aesar | J66429 | Minocycline hydrochloride, 890-950 μg/mg | 13614-98-7 | 1g | $359 | 2024-03-01 | Buy |
Minocycline hydrochloride Chemical Properties,Uses,Production
Description
Minocycline HCl (13614-98-7) displays antiapoptotic, anti-inflammatory1 activity. Prevents neuropathic pain in a rat sciatic nerve injury model.1 Reduces MMP-9 activity.2 Attenuates disease severity in mouse models of multiple sclerosis.3 Displays neuroprotective activity.4 Minocycline HCl may be effective in methotrexate-induced lung fibrosis.5?Orally active and brain penetrant.
Chemical Properties
Yellow Crystalline Powder
Originator
Minocin,Lederle ,US,1971
Uses
antiinflammatory
Uses
Second generation tetracycline antibiotic. Antibacterial.
Uses
Minocycline hydrochloride is a salt prepared from minocycline, taking advantage of the two basic dimethylamino groups which protonate and readily form a salt from hydrochloric acid solutions. The hydrochloride is the preferred formulation for pharmaceutical applications. Like all tetracyclines, minocycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal sub-units, blocking protein synthesis.
Manufacturing Process
Preparation of 7-(N,N'-Dicarbobenzyloxyhydrazino)-6-Demethyltetracycline: A1.0 g portion of 6-demethyltetracycline was dissolved in a mixture of 9.6 ml oftetrahydrofuran and 10.4 ml of methanesulfonic acid at -10°C. The mixturewas allowed to warm to 0°C. A solution of 0.86 g of dibenzyl azodicarboxylatein 0.5 ml of tetrahydrofuran was added dropwise and the mixture was stirredfor 2 hours while the temperature was maintained at 0°C. The reactionmixture was added to ether. The product was filtered off, washed with etherand then dried. The 7-(N,N'-dicarbobenzyloxyhydrazino)-6-demethyltetracycline was identified by paper chromatography.
Reductive Methylation of 7-(N,N'-Dicarbobenzyloxyhydrazino)-6-Demethyl-6-Deoxytetracycline to 7-Dimethylamino-6-Demethyl-6-Deoxytetracycline: Asolution of 100 mg of 7(N,N'-dicarbobenzyloxyhydrazino)-6-demethyl-6-deoxytetracycline in 2.6 ml of methanol, 0.4 ml of 40% aqueous ormaldehyde solution and 50 mg of 5% palladium on carbon catalyst washydrogenated at room temperature and two atmospheres pressure. Uptake ofthe hydrogen was complete in 3 hours. The catalyst was filtered off and thesolution was taken to dryness under reduced pressure. The residue wastriturated with ether and then identified as 7-dimethylamino-6-demethyl-6-deoxytetracycline by comparison with an authentic sample, according to USPatent 3,483,251.
brand name
Dynacin (Medicis); Minocin (Lederle); Minocin (Triax); Solodyn (Medicis).
Therapeutic Function
Antibiotic
General Description
Minocycline, 7-dimethylamino-6-demethyl-6-deoxytetracycline(Minocin, Vectrin), the most potent tetracycline currentlyused in therapy, is obtained by reductive methylationof 7-nitro-6-demethyl-6-deoxytetracycline. It was releasedfor use in the United States in 1971. Because minocycline,like doxycycline, lacks the 6-hydroxyl group, it is stablein acids and does not dehydrate or rearrange to anhydroor lactone forms. Minocycline is well absorbed orally togive high plasma and tissue levels. It has a very long serumhalf-life, resulting from slow urinary excretion and moderateprotein binding. Doxycycline and minocycline, alongwith oxytetracycline, show the least in vitro calcium bindingof the clinically available tetracyclines. The improved distributionproperties of the 6-deoxytetracyclines have been attributedto greater lipid solubility.
Perhaps the most outstanding property of minocyclineis its activity toward Gram-positive bacteria, especiallystaphylococci and streptococci. In fact, minocycline hasbeen effective against staphylococcal strains that are resistantto methicillin and all other tetracyclines, includingdoxycycline. Although it is doubtful that minocyclinewill replace bactericidal agents for the treatment of lifethreateningstaphylococcal infections, it may become auseful alternative for the treatment of less serious tissueinfections. Minocycline has been recommended for thetreatment of chronic bronchitis and other upper respiratorytract infections. Despite its relatively low renal clearance,partially compensated for by high serum and tissuelevels, it has been recommended for the treatment of urinary tract infections. It has been effective in the eradicationof N. meningitidis in asymptomatic carriers.
Biochem/physiol Actions
Minocycline is a broad spectrum antibiotic with bacteriostatic function. Minocycline has anti-inflammatory properties. Minocycline inhibits lipopolysaccharide mediated inflammatory cytokine tumour necrosis factor (TNF-α) secretion by macrophages. Minocycline inhibits macrophage proliferation in a dose dependent manner. Minocycline inhibits neuroinflammation in pre-plaque of Alzheimer′s disease-like amyloid pathology through inhibition of key inflammatory enzymes like inducible nitric oxide synthase (iNOS), matrix metalloproteinase 9 (MMP-9) and 5-lipoxygenase. Minocycline inhibits endothelial cell proliferation and angiogenesis. Minocycline exhibits anti-tumor activity in glioma by inhibiting membrane type 1 matrix metalloproteinase (MT1-MMP). Minocycline increases cognition and neuronal differentiation. zMinocycline effectively reduces neuropathic pain by increasing the functions of nociceptin/orphanin FQ.
Side effects
Common side effects of Minocycline hydrochloride include: nausea, vomiting, diarrhoea, dizziness, lightheadedness or spinning sensation. Individuals may experience symptoms of serious adverse reactions such as gingival hyperpigmentation, pain/difficulty swallowing, tinnitus or hearing loss, joint stiffness/pain/swelling, nephrotoxicity (elevated urea nitrogen, interstitial nephritis), hepatotoxicity (hyperbilirubinaemia, hepatic cholestasis, elevated liver enzymes, fatal hepatic failure, and jaundice), and hypersensitivity reactions. It rarely causes elevated pressure around the brain (intracranial hypertension - IH). The risk of this side effect is greater in women of childbearing age who are overweight or who have had IH in the past. Serious intestinal disorders including: non-stop diarrhoea, abdominal or stomach pain/cramps, and blood/mucus in the stool are less common.
Veterinary Drugs and Treatments
Minocycline may be useful for treating Brucellosis (in combination with aminoglycosides), Lyme disease, and certain nosocomial infections where other more commonly used drugs are ineffective. It has been investigated as adjunctive therapy for treating hemangiosarcomas, but early results have been disappointing.
storage
+4°C
References
1) Padi and Kulkarni (2008), Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms; Eur. J. Pharmacol., 601 79 2) Dziembowska et al. (2013), High MMP-9 activity levels in fragile X syndrome are lowered by minocycline; Am. J. Med. Genet. A, 161A 1897 3) Brundula et al. (2002), Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis; Brain., 125 1297 4) Tikka et al. (2001), Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia; J. Neurosci., 21 2580 5) Kalemci et al. (2013), The efficacy of minocycline against methotrexate-induced pulmonary fibrosis in mice; Eur. Rev. Med. Pharmacol. Sci., 17 3334
Minocycline hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Biopole Pharmatech Co., Ltd. | +8615151475053 | biopole@163.com | China | 37 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8811 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Guangzhou Tengyue Chemical Co., Ltd. | +86-86-18148706580 +8618826483838 | evan@tyvovo.com | China | 148 | 58 |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 467 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Shengyang Water Conservancy Engineering Co., Ltd. | +8615373025980 | clara@hbshengyang.com | China | 874 | 58 |
View Lastest Price from Minocycline hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | Minocycline hydrochloride
13614-98-7
|
US $200.00-85.00 / kg | 1kg | 99% | 20ton | Hebei Zhuanglai Chemical Trading Co.,Ltd | |
 |
2024-11-22 | Minocycline hydrochloride
13614-98-7
|
US $0.00 / g/Bag | 100g | 890-950ug/mg; USP41 | 400kg/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Minocycline hydrochloride
13614-98-7
|
US $62.00-38.00 / mg | 99.89% | 10g | TargetMol Chemicals Inc. |
-

- Minocycline hydrochloride
13614-98-7
- US $200.00-85.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co.,Ltd
-

- Minocycline hydrochloride
13614-98-7
- US $0.00 / g/Bag
- 890-950ug/mg; USP41
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Minocycline hydrochloride
13614-98-7
- US $62.00-38.00 / mg
- 99.89%
- TargetMol Chemicals Inc.
13614-98-7(Minocycline hydrochloride)Related Search:
1of4