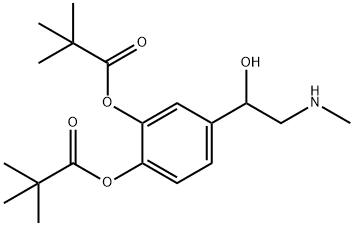
Dipivefrine
- CAS No.
- 52365-63-6
- Chemical Name:
- Dipivefrine
- Synonyms
- K30081;K 30081;K-30081;DIPIVEFRIN;dipiverfrin;dipivefrine;Dipivefrina;Dipivefrinum;Dipivefrine-d3;Dipivefrine USP/EP/BP
- CBNumber:
- CB6676807
- Molecular Formula:
- C19H29NO5
- Molecular Weight:
- 351.44
- MDL Number:
- MFCD00673243
- MOL File:
- 52365-63-6.mol
- MSDS File:
- SDS
| Melting point | 146-147° |
|---|---|
| pka | pKa 8.40(RT) (Uncertain) |
| FDA UNII | 8Q1PVL543G |
| ATC code | S01EA02 |
Dipivefrine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0009084 | DIPIVEFRIN 95.00% | 52365-63-6 | 5MG | $503.88 | 2021-12-16 | Buy |
| AvaChem | 1695B | Dipivefrin | 52365-63-6 | 10mg | $79 | 2021-12-16 | Buy |
| AvaChem | 1695B | Dipivefrin | 52365-63-6 | 50mg | $169 | 2021-12-16 | Buy |
Dipivefrine Chemical Properties,Uses,Production
Originator
Propine,Allergan,W. Germany,1978
Uses
Adrenergic (ophthalmic).
Definition
ChEBI: Dipivefrin is the dipivalate ester of (+-)-epinephrine (racepinephrine). A pro-drug of epinephrine, the hydrochloride is used topically as eye drops to reduce intra-ocular pressure in the treatment of open-angle glaucoma or ocular hypertension. It has a role as a prodrug, an adrenergic agonist, a sympathomimetic agent, an antiglaucoma drug and an ophthalmology drug. It is a member of ethanolamines and a pivalate ester. It is functionally related to an adrenaline. It is a conjugate base of a dipivefrin(1+).
Manufacturing Process
First, 0.27 mol of α-chloro-3',4'-dihydroxyacetophenone are dissolved in 200
ml methanol with warming. Next, 100 ml of a 40% aqueous solution of
methylamine is slowly added and the mixture stirred at 50°C to 55°C for 2
hours. The reaction mixture is then stirred an additional 24 hours at room
temperature.
The crude product separates as a solid from the reaction medium and is
recovered by filtration, and it is then washed thoroughly with ether and
dissolved in 350 ml 1 N HCl. Then, approximately 250 ml of the aqueous
solvent is removed with a rotary evaporator and the evaporation residue
combined with 125 ml methanol and filtered through decolorizing charcoal.
The product is precipitated as the HCl salt by the addition of 7 parts of
acetone. The resulting crystalline material is removed by filtration dried at
40°C with vacuum, and has a melting point of about 242°C and is used
without further purification.
Next, 25.3 g, 0.125 mol, of the above product are dissolved in 250 ml ethyl
acetate and 0.125 mol perchloric acid as a 70% aqueous solution is slowly
added thereto with continuous stirring. Then, an excess of pivaloyl chloride,
280 ml, is added and the mixture slowly warmed to reflux temperature. The
reaction mixture is refluxed for about 5 hours and allowed to cool to room
temperature with continuous stirring. The product is precipitated as the
perchlorate salt by the addition of perchloric acid, HClO4, in 500 ml ether. The
product is isolated and purified by dissolving in 75 ml acetone and
precipitating it with 150 to 200 ml of water.
To 20 g of the above compound dissolved in 300 ml 95% ethanol in a Parr
reaction vessel is added 1.5 g Adams catalyst, platinum dioxide, and the
mixture shaken under hydrogen at 50 psi for 1 hour at ambient temperature.
The mixture is then filtered and the ethanol removed on a standard rotary
evaporator. The resulting oil is dissolved in 200 ml ether and slowly added to
1,200 ml ether with continuous stirring. The product separates as crystals
which are removed after 15 to 30 minutes by filtration. The compound melts
at 146°C to 147°C and needs no further purification.
Therapeutic Function
Adrenergic (ophthalmic)
General Description
Toovercome several of the pharmacokinetic and pharmaceuticalshortcomings of E as an ophthalmic agent, the prodrugapproach has been successfully applied. Dipivefrin is a prodrugof E that is formed by the esterification of the catecholOH groups of E with pivalic acid. Most of the advantages ofthis prodrug over E stem from improved bioavailability. Thegreatly increased lipophilicity allows much greater penetrabilityinto the eye through the corneal epithelial andendothelial layer. The stroma in between requires hydrophilicityfor penetration. Dipivefrin has that, too, due tothe 1-OH group and cationic nitrogen (the eyedrops containthe hydrochloride [HCl] salt). This dual solubility permitsmuch greater penetrability into the eye than the very hydrophilicE hydrochloride. Increased DOA is also achievedbecause the drug is resistant to the metabolism by COMT.In addition to its increased in vivo stability, it is also lesseasily oxidized by air due to the protection of the catecholOH groups. This high bioavailability and in vivo and in vitrostability translate into increased potency such that the 0.1%ophthalmic solution is approximately equivalent to a 2% Esolution. After its absorption, it is converted to E by esterasesslowly in the cornea and anterior chamber.Dipivefrin also offers the advantage of being less irritatingto the eye than E.
Dipivefrine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32072 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49734 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52925 | 58 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23912 | 58 |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2123 | 70 |
| Shanghai Send Pharmaceutical Technology Co., Ltd. | 021-58088081 Q3382968513 | hailey@shsendpharm.com | China | 3130 | 55 |
| Shanghai Resuperpharmtech Co., Ltd. | 02150565560; 17321237488 | kevin@resuperpharmtech.com | China | 360 | 58 |
| BOC Sciences | -- | info@bocsci.com | USA | 0 | 65 |
View Lastest Price from Dipivefrine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-17 | Dipivefrine
52365-63-6
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd |
-

- Dipivefrine
52365-63-6
- US $1.10 / g
- 99.0% min
- Shaanxi Dideu Medichem Co. Ltd
52365-63-6(Dipivefrine)Related Search:
1of4