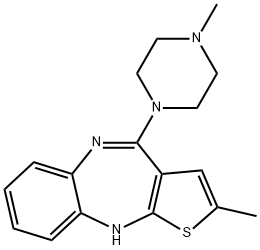
Olanzapine
- CAS No.
- 132539-06-1
- Chemical Name:
- Olanzapine
- Synonyms
- ZYPREXA;OLANZAPINE FORM I;2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine, LY-170053, Zyprexa;Larmac;Oleanz;Olanzne;Integrol;Olanzapine (200 mg);2-Methyl-4-(4-Methylpiperazin-1-yl)-10H-benzo[b]thieno[2,3-e][1,4]diazepine;Oltal
- CBNumber:
- CB6755515
- Molecular Formula:
- C17H20N4S
- Molecular Weight:
- 312.43
- MDL Number:
- MFCD00866702
- MOL File:
- 132539-06-1.mol
- MSDS File:
- SDS
| Melting point | 195°C |
|---|---|
| Boiling point | 476.0±55.0 °C(Predicted) |
| Density | 1.32±0.1 g/cm3(Predicted) |
| Flash point | 2℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: >15mg/mL |
| form | powder |
| pka | 7.78±0.20(Predicted) |
| color | yellow |
| Merck | 14,6822 |
| BRN | 7655141 |
| BCS Class | 2 |
| CAS DataBase Reference | 132539-06-1(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | olanzapine; Zyprexa |
| FDA UNII | N7U69T4SZR |
| NCI Drug Dictionary | olanzapine |
| ATC code | N05AH03 |
| NIST Chemistry Reference | Olanzapine(132539-06-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P305+P351+P338-P332+P313-P337+P313 | |||||||||
| Hazard Codes | Xi,Xn,F | |||||||||
| Risk Statements | 36/38-36-20/21/22-11 | |||||||||
| Safety Statements | 26-36/37-16 | |||||||||
| RIDADR | UN 2811 6.1 / PGIII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XJ9007750 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29349990 | |||||||||
| Toxicity | human,TDLo,oral,16560ug/kg/12 (16.56mg/kg),Journal of Clinical Psychiatry. Vol. 60, Pg. 767, 1999. | |||||||||
| NFPA 704 |
|
Olanzapine price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | O-024 | Olanzapine solution 1.0?mg/mL in acetonitrile, ampule of 1?mL, certified reference material, Cerilliant? | 132539-06-1 | 1mL | $95.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1478301 | Olanzapine United States Pharmacopeia (USP) Reference Standard | 132539-06-1 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | O0393 | Olanzapine >98.0%(GC)(T) | 132539-06-1 | 1g | $90 | 2024-03-01 | Buy |
| TCI Chemical | O0393 | Olanzapine >98.0%(GC)(T) | 132539-06-1 | 5g | $287 | 2024-03-01 | Buy |
| Cayman Chemical | 11937 | Olanzapine ≥98% | 132539-06-1 | 25mg | $32 | 2024-03-01 | Buy |
Olanzapine Chemical Properties,Uses,Production
Antipsychotics
Olanzapine also known as olanzapine, olanzapine Oran, is a common antipsychotic drugs, clinically used to control schizophrenia, bipolar mania and agitation symptoms of dementia, it can significantly improve schizophrenia negative (for example: apathy, emotional and social withdrawal, poverty of speech), positive symptoms (such as: delusions, hallucinations, thought disorder, hostility and suspicion), and it may also relieve common secondary schizophrenia and related disorders affective symptoms.
Good oral absorption, it needs 5-8 hours to reach the peak plasma concentration, and it is not affected by eating, it is metabolized in the liver through a combination of oxidation ; the major circulating metabolite is the 10-N-glucuronide.
Animal experiments show that olanzapine displays affinity on multiple receptors 5-HT, dopamine D, α-adrenergic, histamine H et , the affinity with 5-HT2 receptor in vitro and in vivo is greater than its affinity for dopamine D2 receptors.
Animal behavior studies show that olanzapine has a 5-HT, dopamine, and cholinergic antagonism effect, and it is consistent with its receptor binding situation.
Electrophysiological studies show that olanzapine selectively reduces the limbic system (A10) dopaminergic neurons discharge, while the effect on the striatal (A9) motor path function is very small. In the reaction below to produce froze dosage levels, it can reduce the conditioned avoidance response. Different with other antipsychotics, olanzapine increases the anxiolytic response in test .
The above information is edited by the chemicalbook of Tian Ye.
Chemical properties
Crystallization from acetonitrile, melting point of 195 ℃.
Uses
Used to control schizophrenia, bipolar mania and other diseases
Production method
4-amino-2-methyl-10H-thieno [2,3-b] [1,5] benzo-diazepin-hydrochloride and N-methylpiperazine in toluene and dimethylsulfoxide , reflux under nitrogen for 20h, to obtain olanzapine.
Description
O lanzapine is classed as an atypical antipsychotic and is considered a firstline agent in the management of newly diagnosed psychosis. It has a similar mechanism of action to classical antipsychotics but with a lower incidence of adverse events. O lanzapine rarely produces hypotension and has less potential for QT prolongation. It is used in the management of delirium in ICU.
Description
Zyprexa was launched in Canada, Germany, the UK and US as an antipsychotic agent. Prepared in three steps via the intermediate diazepinone, it is an atypical antipsychotic with a high affinity for dopaminergic and serotonergic receptors. Specifically, olazapine is a potent 5-HT2/D2 antagonist with anticholinergic activity. It has a greater antagonistic effect at 5-HT2a than at dopamine D2 receptors and in vivo is clozapine-like. Thus it is less likely to produce extrapyramidal side effects and does not produce any granulocytopenia. Its 10 metabolic products are all inactive.
Chemical Properties
Yellow Crystalline Powder
Originator
Lilly (USA)
Uses
intermediate for Imidacloprid, Indobufen, Nitroguanidine, Nalorphine, Tazarotene, Trovafloxacin
Uses
A serotonin (5-HT2) and dopamine (D1/D2) receptor antagonist with anticholinergic activity. Antipsychotic.
Uses
anti-ulcer, gastrointestinal-emptying agent enhances motility in the upper gastrointestinal tract
Definition
ChEBI: A benzodiazepine that is 10H-thieno[2,3-b][1,5]benzodiazepine substituted by a methyl group at position 2 and a 4-methylpiperazin-1-yl group at position 4.
Manufacturing Process
1. 2-Amino-5-methylthiophene-3-carbonitrile
A mixture of sulphur (217.8 g, 6.79 mol), propionaldehyde (472.5 g, 587 mL, 8.13 mol) and dimethylformamide (1350 m) was placed in a 5 liter flangenecked flask fitted with air stirrer, air condenser, thermometer and dropping funnel. Triethylamine (576 mL, 4.13 mol) was added dropwise over 30 minutes to the cooled stirred reaction mixture whilst maintaining the pot temperature between 5°-10°C with an ice-bath. After addition was complete the pot was allowed to warm up to 18°C over 50 minutes, keeping the mixture well stirred. Then a solution of malononitrile (450 g, 6.8 mol) in dimethylformamide (900 mL) was added dropwise over 70 minutes keeping the pot temperature around 20°C throughout the addition. After addition was complete the mixture was stirred at 15°-20°C for a further 45 minutes then sampled for TLC. The mixture was then poured onto ice (4 liters)/water (8 liters) with stirring and this caused the required product to precipitate. After 10 minutes the stirrer was switched off and the solid allowed to settle. The aqueous liquor was decanted away and the solid isolated by filtration. The isolated solid was well washed with water (de-ionised, 4 liters), then dried over night in vacuo at 70°-75°C to give the title compound (585 g), m.p. 100°C.
2. 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile
To a stirred slurry of sodium hydride (14.4 g, 50% dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoronitrobenzene (28.2 g, 0.2 mol) and 2-amino-5methylthiophene3-carbonitrile (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25°C for 24 hours, poured onto cracked ice and extracted into dichloromethane (3 times 500 mL). The combined extracts were washed with 2 N hydrochloric acid (2 times 200 mL), water (2 times 200 mL), dried over magnesium sulphate and the solvent removed under reduced pressure. The residue was crystallised from ethanol to give the title compound, (35.2 g), m.p. 99°-102°C.
3. 4-Amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine, hydrochloride
To a stirred slurry of 2-(2-nitroanilino)-5-methylthiophene-3-carbonitrile (3 g, 0.011 mol) in ethanol (35 mL) at 50°C was added, over 10 minutes, a solution of anhydrous stannous chloride (6.95 g, 0.037 mol) in hydrochloric acid (26 mL, 5 M). The mixture was stirred under reflux for 1 hour, concentrated under reduced pressure and allowed to crystallise over night at 5°C. The salt was filtered, washed with a small amount of water, dried (4.3 g) m.p. >250°C, and used without further purification in the next stage.
4. 2-Methyl-10-(4-methyl-1-piperazinyl)-4H-thieno[2,3-b][1,5]benzodiazepine
Crude 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine, hydrochloride (4.3 g) was refluxed in a mixture of N-methylpiperazine (15 mL), dimethylsulfoxide (20 mL) and toluene (20 mL) under a nitrogen atmosphere for 20 hours. The mixture was cooled to ca. 50°C, water (20 mL) added, and the product allowed to crystallise at 5°C over night. The product was filtered and crystallised from acetonitrile (30 mL) to give the title compound (1.65 g) m.p. 195°C. The structure of the compound was confirmed spectroscopically
brand name
Zyprexa (Lilly).
Therapeutic Function
Antipsychotic
General Description
Olanzapine, 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5] benzodiazepine (Zyprexa), isa yellow crystalline solid that is essentially insoluble in water.Olanzapine orally disintegrating tablets (Zyprexa Zydis) is asolid dosage form that immediately disintegrates when exposedto saliva. This product is useful for elderly patients whohave difficulty in swallowing. An injectable form, olanzapinefor injection (Zyprexa IM), is indicated for agitation associatedwith schizophrenia or bipolar I mania. Olanzapine iscombined with fluoxetine (Symbyax) for use in depressionthat is associated with bipolar I disorder. Peak concentrationsof oral olanzapine are reached at 6 hours after oral administration,and absorption of the compound is not affected byfood.
Olanzapine binds with high affinity at DA D2, 5-HT2A,5-HT2C, 5-HT6,α1, and H1 histamine receptors. The effectsof olanzapine for the treatment of schizophrenia are presumablymediated through antagonism at D2 and 5-HT2Areceptors.The use of olanzapine in acute maniathat is associated with bipolar I disorder is thought to bemediated by antagonism at D2 and other monamine receptors.Additionally, olanzapine is postulated to produce itsmood stabilizing and antidepressant effects through 5-HT2Areceptor blockade and increased cortical DA and NE concentrations.Several studies have investigated the effectof olanzapine-induced weight gain and new-onset type 2diabetes.In a comparison study with risperidone,olanzapine was shown to have a greater risk of producingdyslipidemia and type 2 diabetes.
Biochem/physiol Actions
Olanzapine is a 5-HT2 serotonin and D1/D2 dopamine receptor antagonist.
Clinical Use
The thienobenzodiazepine olanzapine is an effective atypical antipsychotic agent that is close in structure to clozapine but has a somewhat different neuropharmacological profile, in that it is a more potent antagonist at dopamine D2 and, especially, serotonin 5-HT2A receptors. Olanzapine is well absorbed after oral administration and is metabolized mainly by CYP1A2 to inactive metabolites, with a variable half-life of approximately 20 to 50 hours.
in vitro
binding studies showed that olanzapine interacted with keyreceptorsof interest in schizophrenia, exihibiting a nanomolar affinity for dopaminergic, serotonergic, alpha 1-adrenergic, and muscarinic receptors [1].
in vivo
olanzapine was a potent antagonist at dareceptorsand 5-ht receptors, but showed weaker activity at alpha-adrenergic and muscarinic receptors [1].administration of olanzapine at 0.5, 3 and 10 mg/kg (s.c.) increased the extracellulardopamine(da) and norepinephrine (ne) levels in all three brain areas in a dose-dependent manner.the increases reached peaks 60-90 min after olanzapine administration and lasted for at least 2 h. the highest da increases in the acb and cpu were induced by olanzapine at 3 mg/kg but at 10 mg/kg in the pfc while the highest ne increase in the pfc (414% ± 40) induced by 10 mg/kg olanzapine [2].in macaque monkeys, olanzapine treatment resulted in an 8-11% reduction in mean fresh brain weights as well as left cerebrum fresh weights and volumes [3].
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias.
Antibacterials: concentration possibly increased by
ciprofloxacin.
Antidepressants: fluvoxamine increases concentration
of olanzapine; increased concentration of tricyclics.
Antiepileptics: antagonism (convulsive threshold
lowered); carbamazepine increases metabolism
of olanzapine; increased risk of neutropenia with
valproate.
Antimalarials: avoid with artemether/lumefantrine.
Antipsychotics: increased risk of ventricular
arrhythmias with risperidone.
Antivirals: concentration reduced by ritonavir -
consider increasing olanzapine dose.
Anxiolytics and hypnotics: increased sedative
effects; increased risk of hypotension, bradycardia
and respiratory depression with IM olanzapine and
parenteral benzodiazepines.
Atomoxetine: increased risk of ventricular
arrhythmias.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Metabolism
Olanzapine is extensively metabolised in the liver, mainly
by direct glucuronidation and by oxidation mediated
through the cytochrome P450 isoenzymes CYP1A2, and,
to a lesser extent, CYP2D6. The 2 major metabolites,
10-N-glucuronide and 4′-N-desmethyl olanzapine,
appear to be inactive.
About 57% of a dose is excreted in the urine, mainly as
metabolites, and about 30% appears in the faeces.
storage
+4°C
References
[1]. bymaster fp1,rasmussen k,calligaro do,nelson dl,delapp nw,wong dt,moore na. in vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug.j clin psychiatry.1997;58suppl 10:28-36.
[2]. li xm1,perry kw,wong dt,bymaster fp. olanzapine increases in vivodopamineand norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum.psychopharmacology (berl).1998 mar;136(2):153-61.
[3]. dorph-petersen ka1,pierri jn,perel jm,sun z,sampson ar,lewis da. the influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys.neuropsychopharmacology.2005 sep;30(9):1649-61.
Olanzapine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 | mack@tongyon.com | China | 2994 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18154 | 58 |
| Guangzhou Tengyue Chemical Co., Ltd. | +86-86-18148706580 +8618826483838 | evan@tyvovo.com | China | 148 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1177 | 58 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 | sales@czwytech.com | CHINA | 904 | 58 |
View Lastest Price from Olanzapine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Olanzapine
132539-06-1
|
US $0.00 / KG | 1KG | 99.0~101.0% | 500KG/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Olanzapine
132539-06-1
|
US $30.00-55.00 / mg | ≥95% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-01 | Olanzapine
132539-06-1
|
US $0.00 / KG | 1KG | 99% | 500000kg | Hebei Weibang Biotechnology Co., Ltd |
-

- Olanzapine
132539-06-1
- US $0.00 / KG
- 99.0~101.0%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Olanzapine
132539-06-1
- US $30.00-55.00 / mg
- ≥95%
- TargetMol Chemicals Inc.
-

- Olanzapine
132539-06-1
- US $0.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd