Lithium oxide
- CAS No.
- 12057-24-8
- Chemical Name:
- Lithium oxide
- Synonyms
- Li2O;Lithia;Kickerite;Lithiumoxid;Oxydilithium;LITHIUM OXIDE;Oxybislithium;dilithiumoxide;oxydedelithium;lithiummonoxide
- CBNumber:
- CB7164125
- Molecular Formula:
- Li2O
- Molecular Weight:
- 29.88
- MDL Number:
- MFCD00016183
- MOL File:
- 12057-24-8.mol
| Melting point | 1427°C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | 2.013 g/mL at 25 °C (lit.) | ||||||||||||||
| form | Powder | ||||||||||||||
| Specific Gravity | 2.013 | ||||||||||||||
| color | White | ||||||||||||||
| Water Solubility | Soluble in water. | ||||||||||||||
| Sensitive | Air & Moisture Sensitive | ||||||||||||||
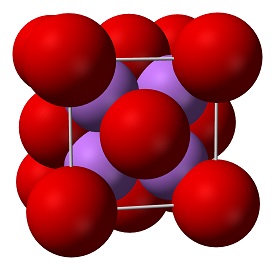
| Crystal Structure | Reverse CaF2 type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Merck | 14,5538 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| CAS DataBase Reference | 12057-24-8(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1 | ||||||||||||||
| FDA UNII | 2ON0O9YI0Q | ||||||||||||||
| NIST Chemistry Reference | Dilithium monoxide(12057-24-8) | ||||||||||||||
| EPA Substance Registry System | Dilithium oxide (12057-24-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P260-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 3262 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OJ6360000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
Lithium oxide price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 374725 | Lithium oxide 97%, powder, 60 mesh | 12057-24-8 | 10g | $107 | 2024-03-01 | Buy |
| Sigma-Aldrich | 374725 | Lithium oxide 97%, powder, 60 mesh | 12057-24-8 | 100g | $561 | 2024-03-01 | Buy |
| Alfa Aesar | 041832 | Lithium oxide, 99.5% (metals basis) | 12057-24-8 | 10g | $76.6 | 2024-03-01 | Buy |
| Alfa Aesar | 041832 | Lithium oxide, 99.5% (metals basis) | 12057-24-8 | 50g | $298 | 2024-03-01 | Buy |
| Strem Chemicals | 03-0343 | Lithium oxide, min. 95% (99.5%-Li) | 12057-24-8 | 25g | $156 | 2024-03-01 | Buy |
Lithium oxide Chemical Properties,Uses,Production
Chemical Properties
Lithium oxide (Li2O) is one of simplest ionic oxides and it is isoelectronic to H2O. Two lithium atoms will each give one electron to the oxygen atom. forms the ionic bond between lithium and oxygen. The formula for lithium oxide is Li2O.
Lithium oxide is very corrosive. It reacts with water to make lithium hydroxide. It is toxic because of its strong alkalinity (being a base).
It is a highly insoluble thermally stable Lithium source suitable for glass, optic and ceramic applications. Lithium oxide is a white solid also known as lithia, it is produced when lithium metal burns in the presence of oxygen. Oxide compounds are not conductive to electricity. However, certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systems. They are compounds containing at least one oxygen anion and one metallic cation.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them. Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating.
Uses
There are no current industrial uses which consume large quantities of lithium oxide.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them.Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating.
Reactions
Lithium oxide reacts with water as it dissolves to form a solution of lithium hydroxide.
Lithium oxide is a strong base and reacts typically with acidic gases and liquids to form lithium salts. At elevated temperatures, lithium oxide also reacts with many solid nonmetal oxides (SiO2, B2O3, etc.) and metal oxides (A12O3, Fe2O3, etc.). High-temperature reactions are the basis for the fluxing action of lithium oxide, lithium hydroxide and lithium carbonate. Care must be taken to avoid the reaction of lithium oxide with reaction vessels at high temperatures.
Preparation
Lithium oxide is prepared by heating lithium metal in dry oxygen above 100°C:
4Li + O2→2Li2O
Another method of preparation that yields pure lithium oxide involves thermal decomposition of lithium peroxide:
2Li2O2→2Li2O + O2
Also, the oxide can be produced by heating the pure lithium hydroxide at 800°C in a vacuum:
2LiOH→Li2O + H2O
Health Hazard
To the best of our knowledge the chemical, physical and toxicological properties of lithium oxide have not been thoroughly investigated and reported.
The toxicity of lithium compounds is a function of their solubility in water. Lithium ion has central nervous system toxicity. The initial effects of lithium exposure are tremors of the hands, nausea, micturition, slurred speech, sluggishness, sleepiness, vertigo, thirst, and increased urine volume. Effects from continued exposure are apathy, anorexia, fatigue, lethargy, muscular weakness, and changes in ecg. Long-term exposure leads to hypothyroidism, leukocytosis, edema, weight gain, polydipsia/polyuria (increased water intake leading to increased urinary output), memory impairment, seizures, kidney damage, shock, hypotension, cardiac arrhythmias, coma, death.
Chemical Properties
finely divided white powder(s) or crusty material; readily absorbs CO2 and H2O from the atmospheric; made by heating LiOH to ~800°C in a vacuum or by thermal decomposition of lithium peroxide; used in ceramics and special glass formulations and in lithium thermal batteries [HAW93] [MER06] [KIR81] [FMC93]
Uses
Ceramics and special glass formulations, carbon dioxide absorbent.Lithium oxide is used as a flux in ceramic glazes, co-dopant with yttria in the zirconia ceramic top coat and as the cathode in the lithium ion batteries, which is used in power electronic devices like mobile phones, laptop computers and battery-powered cars. It is also used to prepare lithium hydroxide and lithium metal by electrolysis.
Uses
Lithium oxide is a strong alkali that absorbs carbon dioxide and water from the atmosphere. It is used in manufacturing ceramics and special types of glass.
Preparation
Industrial and laboratory preparations. Only small volumes of material are prepared
industrially. Both industrial and laboratory preparations require the thermal decomposition
of lithium peroxide or of lithium hydroxide.
Lithium peroxide, Li202 , is converted to lithium oxide, Li20, and oxygen by heating
to 450° in a stream of helium gas.
Thermal dehydration of lithium hydroxide is carried out at 675°C±10° under vacuum
in a nickel container lined with silver foil.
Lithium carbonate may be converted to lithium oxide and carbon dioxide by heating
the material to 700°C under vacuum in a platinum boat.
Industrial uses. There are no current industrial uses which consume large quantities of
lithium oxide.
Lithium oxide reacts with water as it dissolves to form a solution of lithium hydroxide.
Lithium oxide is a strong base and reacts typically with acidic gases and liquids to form
lithium salts. At elevated temperatures, lithium oxide also reacts with many solid nonmetal
oxides (Si02, B2O3, etc.) and metal oxides (A1203 , Fe2C>3, etc.). High-temperature
reactions are the basis for the fluxing action of lithium oxide, lithium hydroxide and
lithium carbonate. Care must be taken to avoid the reaction of lithium oxide with reaction
vessels at high temperatures.
General Description
Lithium oxide is a white crystalline solid. Its a strong base. It reacts with water and forms lithium hydroxide.
Structure and conformation
Solid lithium oxide adopts an antifluorite structure with four-coordinated Li+ centers and eight-coordinated oxides.The ground state gas phase Li2O molecule is linear with a bond length consistent with strong ionic bonding.VSEPR theory would predict a bent shape similar to H2O.
Lithium oxide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18152 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 2904 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 | sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
Related articles
- Lithium Oxide: A Critical Compound in Modern Chemistry and Technology
- Lithium oxide, with the chemical formula Li?O, is a white, inorganic solid compound that is formed by the reaction of lithium ....
- Sep 26,2024
View Lastest Price from Lithium oxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-17 | Lithium oxide
12057-24-8
|
US $500.00-30000.00 / kg | 1kg | 99.5% Min. | 1000kg | Henan Alfa Chemical Co., Ltd | |
 |
2024-10-25 | Lithium oxide
12057-24-8
|
US $1.00 / KG | 1KG | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-25 | Lithium oxide
12057-24-8
|
US $0.00-0.00 / KG | 1KG | 99% | 500000kg | Hebei Weibang Biotechnology Co., Ltd |
-

- Lithium oxide
12057-24-8
- US $500.00-30000.00 / kg
- 99.5% Min.
- Henan Alfa Chemical Co., Ltd
-

- Lithium oxide
12057-24-8
- US $1.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Lithium oxide
12057-24-8
- US $0.00-0.00 / KG
- 99%
- Hebei Weibang Biotechnology Co., Ltd