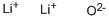산화 리튬
|
|
산화 리튬 속성
- 녹는점
- 1427°C
- 밀도
- 2.013 g/mL at 25 °C (lit.)
- 물리적 상태
- 가루
- Specific Gravity
- 2.013
- 색상
- 하얀색
- 수용성
- 물에 용해됩니다.
- 감도
- Air & Moisture Sensitive
- Crystal Structure
- Reverse CaF2 type
- crystal system
- Cube
- Merck
- 14,5538
- Space group
- Fm3m
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.463 0.463 0.463 90 90 90 0.0993
- CAS 데이터베이스
- 12057-24-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34 | ||
| 안전지침서 | 26-36/37/39-45 | ||
| 유엔번호(UN No.) | UN 3262 8/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | OJ6360000 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| 기존화학 물질 | KE-10991 |
산화 리튬 C화학적 특성, 용도, 생산
화학적 성질
finely divided white powder(s) or crusty material; readily absorbs CO2 and H2O from the atmospheric; made by heating LiOH to ~800°C in a vacuum or by thermal decomposition of lithium peroxide; used in ceramics and special glass formulations and in lithium thermal batteries [HAW93] [MER06] [KIR81] [FMC93]용도
Lithium oxide is a strong alkali that absorbs carbon dioxide and water from the atmosphere. It is used in manufacturing ceramics and special types of glass.제조 방법
Industrial and laboratory preparations. Only small volumes of material are prepared industrially. Both industrial and laboratory preparations require the thermal decomposition of lithium peroxide or of lithium hydroxide.Lithium peroxide, Li202 , is converted to lithium oxide, Li20, and oxygen by heating to 450° in a stream of helium gas.
Thermal dehydration of lithium hydroxide is carried out at 675°C±10° under vacuum in a nickel container lined with silver foil.
Lithium carbonate may be converted to lithium oxide and carbon dioxide by heating the material to 700°C under vacuum in a platinum boat.
Industrial uses. There are no current industrial uses which consume large quantities of lithium oxide.
Lithium oxide reacts with water as it dissolves to form a solution of lithium hydroxide. Lithium oxide is a strong base and reacts typically with acidic gases and liquids to form lithium salts. At elevated temperatures, lithium oxide also reacts with many solid nonmetal oxides (Si02, B2O3, etc.) and metal oxides (A1203 , Fe2C>3, etc.). High-temperature reactions are the basis for the fluxing action of lithium oxide, lithium hydroxide and lithium carbonate. Care must be taken to avoid the reaction of lithium oxide with reaction vessels at high temperatures.
일반 설명
Lithium oxide is a white crystalline solid. Its a strong base. It reacts with water and forms lithium hydroxide.산화 리튬 준비 용품 및 원자재
원자재
준비 용품
산화 리튬 공급 업체
글로벌( 223)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 |
mina@chuanghaibio.com | China | 18137 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 |
sales@zhuoerchem.com | China | 2904 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 |
abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12809 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29858 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Richest Group Ltd | 18017061086 |
oled@richest-group.com | CHINA | 5600 | 58 |