탄산, 리튬 염 C화학적 특성, 용도, 생산
화학적 성질
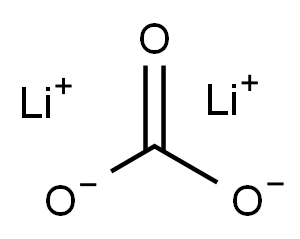
Lithium carbonate is a white hygroscopic powder.
물리적 성질
White monoclinic crystals; refractive index 1.428; density 2.11 g/cm
3; melts at 723°C; decomposes at 1,310°C; low solubility in water (1.54 g/100g) at 0°C; 1.32 g//100g at 20°C), solubility decrease with temperature (0.72g/100g at 100°C); insoluble in acetone and ethanol.
용도
Lithium carbonate is used as a compound for producing metallic
lithium. Lithium carbonate is the result of treating the mineral spodumene with sulfuric
acid and then adding calcium carbonate. It is used as an antidepressant.
Indications
Lithium inhibits thyroidal incorporation of I
- into Tg, as
well as the secretion of thyroid hormones, but it does
not inhibit the activity of the Na
+-I
- symporter or the
accumulation of I
- within the thyroid. Lithium offers no
particular advantage over drugs of the thionamide class
but may be employed for temporary control of thyrotoxicosis
in patients who are allergic to both thionamides
and iodide.
제조 방법
Lithium carbonate is prepared by the precipitation of lithium
ion by carbonate ion from an aqueous solution. Still another process, which is carried out on a smaller
scale, is the reaction of a solution of lithium hydroxide with carbon dioxide gas. Lithium
carbonate precipitates and is recovered from the supernatant solution.
정의
lithium carbonate: A white solid,Li2CO3; r.d. 2.11; m.p. 723°C; decomposesabove 1310°C. It is producedcommercially by treating the ore with sulphuric acid at 250°C andleaching the product to give a solutionof lithium sulphate. The carbonateis then obtained by precipitationwith sodium carbonate solution.Lithium carbonate is used in the preventionand treatment of manicdepressivedisorders. It is also usedindustrially in ceramic glazes.
일반 설명
Lithiumcarbonate (Eskalith, Lithane) and lithium citrate(Cibalith-S) are the salts commercially available in theUnited States.
반응 프로필
A base. Decomposed by acids with the evolution of carbon dioxide. Fluorine burns fiercely on contact with Lithium carbonate.
부작용
Drowsiness, dizziness, tiredness, increased thirst, increased frequency of urination, weight gain, and mildly shaking hands (fine tremor) may occur. These should go away as your body adjusts to the medication.
This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
Safety Profile
Human carcinogenic
data. Poison by intraperitoneal and
intravenous routes. Moderately toxic by
ingestion and subcutaneous routes. Human
systemic effects by ingestion: toxic
psychosis, tremors, changes in fluid intake,
muscle weakness, increased urine volume,
nausea or vomiting, allergic dermatitis.
Human reproductive effects by ingestion:
effects on newborn, including Apgar score
changes and other neonatal measures or
effects. Human teratogenic effects by
ingestion: developmental abnormalities of
the cardiovascular system, central nervous
system, musculoskeletal and gastrointestinal
systems. An experimental teratogen.
Experimental reproductive effects.
Experimental carcinogen producing
leukemia and thyroid tumors. Human
mutation data reported. Used in the
treatment of manic-depressive psychoses.
Incompatible with fluorine. See also
LITHIUM COMPOUNDS.
잠재적 노출
Lithium carbonate is used in treatment
of manic-depressive psychoses; to make ceramics and porcelain glaze; varnishes, dyes, pharmaceuticals, coating of
arc-welding electrodes; battery alloys; nucleonics, luminescent paints; glass ceramics; lubricating greases; in aluminum production
운송 방법
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required
Purification Methods
Crystallise it from water. Its solubility decreases as the temperature is raised. The solubility in H2O is 1.3% at ~10o, and 0.7% at ~100o. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963, Caley & Elving Inorg Synth I 1 1939.]
비 호환성
The aqueous solution is a strong base.
Reacts violently with acids, powdered calcium and fluorine.Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong acids, powdered calcium,
fluorine, moisture. Corrodes aluminum, copper, zinc.
탄산, 리튬 염 준비 용품 및 원자재
원자재
준비 용품