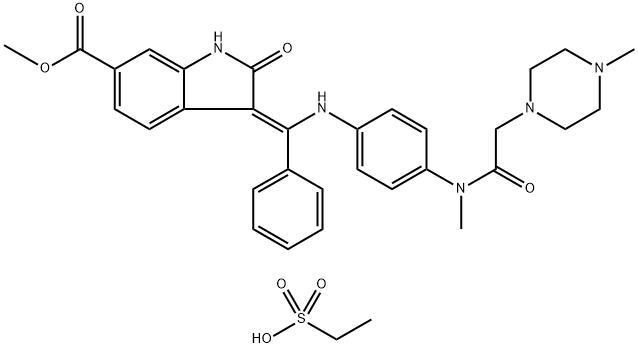
Nintedanib
- CAS No.
- 656247-17-5
- Chemical Name:
- Nintedanib
- Synonyms
- Intedanib;Nintedanib (BIBF 1120);INTEDANIB BASE;methyl (3Z)-3-{[(4-{methyl[(4-methylpiperazin-1-yl)acetyl]amino}phenyl)amino](phenyl)methylidene;(3Z)-3-[[4-[methyl-[2-(4-methylpiperazin-1-yl)acetyl]amino]anilino]-phenylmethylidene]-2-oxo-1H-indole-6-carboxylate;methyl (3Z)-2-hydroxy-3-[({4-[N-methyl-2-(4-methylpiperazin-1-yl)acetamido]phenyl}amino)(phenyl)methylidene]-3H-indole-6-carboxylate;100837;Nidanib;NTEDANIB;Vargatef
- CBNumber:
- CB72510401
- Molecular Formula:
- C31H33N5O4
- Molecular Weight:
- 539.64
- MDL Number:
- MFCD11974012
- MOL File:
- 656247-17-5.mol
| Melting point | >237oC (dec.) |
|---|---|
| Boiling point | 742.2±60.0 °C(Predicted) |
| Density | 1.284±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Yellow solid. |
| pka | 11.06±0.20(Predicted) |
| color | Light Yellow to Light Green |
| InChIKey | XZXHXSATPCNXJR-ZIADKAODSA-N |
| SMILES | C(/C1C=CC=CC=1)(=C1\C(NC2=CC(C(=O)OC)=CC=C\12)=O)\NC1C=CC(N(C)C(=O)CN2CCN(C)CC2)=CC=1 |
| FDA UNII | G6HRD2P839 |
| NCI Drug Dictionary | nintedanib |
| ATC code | L01EX09 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 | |||||||||
| NFPA 704 |
|
Nintedanib price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 11022 | BIBF 1120 ≥95% | 656247-17-5 | 10mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 11022 | BIBF 1120 ≥95% | 656247-17-5 | 50mg | $109 | 2024-03-01 | Buy |
| Cayman Chemical | 11022 | BIBF 1120 ≥95% | 656247-17-5 | 100mg | $193 | 2024-03-01 | Buy |
| Cayman Chemical | 11022 | BIBF 1120 | 656247-17-5 | 250mg | $426 | 2024-03-01 | Buy |
| Tocris | 7049 | Nintedanib ≥98%(HPLC) | 656247-17-5 | 10 | $59 | 2021-12-16 | Buy |
Nintedanib Chemical Properties,Uses,Production
Description
Nintedanib(656247-17-5) is an oral administrated triple tyrosine kinase inhibitors developed by Boehringer Ingelheim, Germany. Its targets include platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and basic fiber (BFGF), being able to also inhibit the activation of MAPK and Akt. At present, it is mainly used for cancer treatment, such as colorectal cancer, ovarian cancer and multiple myeloma. The study regarding to the respiratory diseases mainly focuses on the clinical treatment of advanced non-small cell lung cancer (NSCLC) and idiopathic pulmonary fibrosis (IPF). In June 2014, Boehringer Ingelheim has announced that the marketing authorization application of Nintedanib for the treatment of Idiopathic Pulmonary Fibrosis (IPF) had been confirmed by the European Medicines Agency (EMA) and been included in the accelerated approval list by the EMA.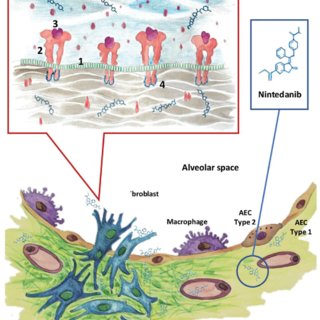
Mechanism of action
Nintedanib(656247-17-5) is a small molecule that targets multiple receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). Nintedanib inhibits the following RTKs: platelet-derived growth factor receptor (PDGFR) α and β, fibroblast growth factor receptor (FGFR) 1-3, vascular endothelial growth factor receptor (VEGFR) 1-3, and Fms-like tyrosine kinase-3 (FLT3). Among them, FGFR, PDGFR, and VEGFR have been implicated in IPF pathogenesis. Nintedanib binds competitively to the adenosine triphosphate (ATP) binding pocket of these receptors and blocks the intracellular signaling which is crucial for the proliferation, migration, and transformation of fibroblasts representing essential mechanisms of the IPF pathology. In addition, nintedanib inhibits the following nRTKs: Lck, Lyn and Src kinases. The contribution of FLT3 and nRTK inhibition to IPF efficacy is unknown.
indications
Nintedanib ( Vargatef ) is an oral triple angiokinase inhibitor which simultaneously inhibits vascular endothelial growth factor receptors (VEGFR), platelet-derived growth factor receptors (PDGFR) and fibroblast growth factor receptors (FGFR) signalling pathways. Growing scientific evidence shows that these three different angiokinase receptors play an important role not only in angiogenesis but also in tumour growth and metastasis.
Nintedanib is currently being investigated in patients with various solid tumours including Phase III studies in advanced NSCLC, colorectal cancer (refractory to standard treatment) and ovarian cancer, and also in Phase II studies in mesothelioma, kidney cancer (renal cell carcinoma) and liver cancer (hepatic cell carcinoma).
In the EU, nintedanib in the treatment of idiopathic pulmonary fibrosis (IPF) has recently received a positive CHMP opinion. The U.S. Food and Drug Administration (FDA) has approved nintedanib capsules under the brand name OFEV for oral use for the treatment of IPF.
Drugbank Description
Nintedanib(656247-17-5) is a drug indicated for the treatment of idiopathic pulmonary fibrosis (IPF) that targets multiple receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (nRTKs). Nintedanib inhibits the following RTKs: platelet-derived growth factor receptor (PDGFR) α and β, fibroblast growth factor receptor (FGFR) 1-3, vascular endothelial growth factor receptor (VEGFR) 1-3, and Fms-like tyrosine kinase-3 (FLT3). Among them, FGFR, PDGFR, and VEGFR have been implicated in IPF pathogenesis. Nintedanib binds competitively to the adenosine triphosphate (ATP) binding pocket of these receptors and blocks the intracellular signaling which is crucial for the proliferation, migration, and transformation of fibroblasts representing essential mechanisms of the IPF pathology. In addition, nintedanib inhibits the following nRTKs: Lck, Lyn and Src kinases. The contribution of FLT3 and nRTK inhibition to IPF efficacy is unknown. Idiopathic pulmonary fibrosis (IPF) is a progressive and ultimately fatal lung disease characterized by a progressive loss of lung function with worsening dyspnoea and cough. Development is thought to be instigated by repetitive lung injury (such as by cigarette smoke, industrial dusts, gastrooesophageal reflux and viral infection) leading to destruction of epithelial alveolar cells. Subsequent dysregulation of the repair process results in the proliferation/migration of fibroblasts and their differentiation into myofibroblasts, abnormal extracellular matrix deposition and excessive collagen accumulation in the lung interstitium and alveolar space, leading to progressive fibrosis and stiffening of the lungs. Vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) mediate various processes, including fibrogenesis and angiogenesis, and are implicated in the pathogenesis of IPF. By blocking substrate binding and downstream signalling cascades, nintedanib interferes with processes active in fibrosis such as fibroblast proliferation, migration and differentiation, and the secretion of extracellular matrix.
References
https://www.drugbank.ca/drugs/DB09079
Uses
It is an indolinone derivative potently blocking VEGF-, PDGF-, and FGF-receptor kinases; an indolinone as triple angiokinase inhibitors. An antitumor agent.
Uses
BIBF1120 (Vargatef) is a potent inhibitor of VEGFR1/2/3, FGFR1/2/3 and PDGFRα/β with IC50 of 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM and 59 nM/65 nM, respectively.
Definition
ChEBI: A member of the class of oxindoles that is a kinase inhibitor used (in the form of its ethylsulfonate salt) for the treatment of idiopathic pulmonary fibrosis and cancer.
Biological Activity
nintedanib (bibf 1120) is an indolinone-derived oral active, triple angiokinase inhibitor of vascular endothelial growth factor receptor (vegfr)1-3, fibroblast growth factor receptor (fgfr)1-3 and platelet-derived growth factor receptor (pdgfr)α/β1. it has shown potent antiangiogenic activity at nanomolar (ic50, 20-100 nmol/l) by blocking these receptor-mediated signaling pathways1,2. nintedanib (bibf 1120) is in clinical development for the treatment of idiopathic pulmonary fibrosis as these receptors have been shown to be potentially involved in the pathogenesis of pulmonary fibrosis3,4. as a novel angiogenesis inhibitor, it is also being widely evaluated in different cancer models and has displayed significant anti-tumor activities by inhibiting tumor blood vessel formation5-7.to further evaluate its antitumor effects on multiple tumors, nintedanib is currently entering several
Clinical Use
Tyrosine kinase inhibitor:
Treatment of locally advanced, metastatic or locally
recurrent non-small cell lung cancer (NSCLC) of
adenocarcinoma tumour histology in combination
with docetaxel
Treatment of Idiopathic Pulmonary Fibrosis (IPF)
Side effects
The most common side effects of Nintedanib in clinical studies were gastrointestinal-related side effects, including nausea (31%), vomiting (23%), diarrhoea (76%), abdominal pain (10%) and decreased appetite (10%). Other side effects were hepatic impairment commonly seen as elevated liver enzymes, weight loss (7 per cent), nasopharyngitis (7 per cent), cough (14 per cent), upper respiratory tract infections (14 per cent), headache (12 per cent), fatigue (14 per cent), skin ulcers (16 per cent) and urinary tract infections (12 per cent).
Reported post-marketing adverse events include gastrointestinal perforation, proteinuria and pancreatitis. In several clinical trials, nidanib has also been found to be associated with an increased risk of bleeding, possibly secondary to VEGFR inhibition. Although serious bleeding and arterial thromboembolic events (e.g., myocardial infarction (MI) and stroke) are rare, bleeding events with serious and fatal consequences have been reported in post-marketing surveillance. Therefore, the use of nidazanib has been associated with an increased risk of bleeding, possibly secondary to VEGFR inhibition. Therefore, patients treated with nidazanib and receiving full anticoagulation therapy need to be closely monitored for adverse bleeding events.
target
VEGFR1/2/3
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antifungals: concentration increased by
ketoconazole.
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid.
Metabolism
Nintedanib is metabolised in the liver, initially by hydrolytic cleavage by esterases and then by glucuronidation by uridine diphosphate glucuronosyltransferase enzymes. Only a minor portion is metabolised by cytochrome P450 isoenzymes, mainly CYP3A4. More than 90% of a dose is eliminated by faecal/biliary excretion.
storage
Store at -20°C
References
[1]hilberg, f. et al. bibf 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. cancer research 68, 4774-4782, doi:10.1158/0008-5472.can-07-6307 (2008).
Nintedanib Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Cangzhou Kangrui Pharma Tech Co. Ltd., | +86-18632776803 +86-13833998158 | cangzhoukangrui@126.com | China | 733 | 58 |
| SyncoZymes (Shanghai) Co., Ltd., | +86-021-68187180-811 +86-13681683526 | lchen@syncozymes.com | China | 188 | 58 |
| HangZhou RunYan Pharma Technology Co.,LTD. | +8618882027439 | sales2@runyanpharma.com | China | 302 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8467 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12837 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5858 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | sales@sdperfect.com | China | 294 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | sales@ichemie.com | China | 998 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 295 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 970 | 58 |
Related articles
- Nintedanib: Uses, Mechanism of Action and Side Effects
- Nintedanib is a small molecule tyrosine kinase inhibitor (TKI) that binds to a family of growth factor receptors and blocks th....
- Apr 18,2024
View Lastest Price from Nintedanib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-04 | Nintedanib
656247-17-5
|
US $0.00 / kg | 1kg | 99% HPLC | 1000kg | HangZhou RunYan Pharma Technology Co.,LTD. | |
 |
2024-11-04 | Nintedanib
656247-17-5
|
US $0.00-0.00 / kg | 1kg | 99%, Single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd | |
 |
2024-11-04 | Nintedanib
656247-17-5
|
US $38.00-64.00 / mg | 99.92% | 10g | TargetMol Chemicals Inc. |
-

- Nintedanib
656247-17-5
- US $0.00 / kg
- 99% HPLC
- HangZhou RunYan Pharma Technology Co.,LTD.
-

- Nintedanib
656247-17-5
- US $0.00-0.00 / kg
- 99%, Single impurity<0.1
- Nanjing Fred Technology Co., Ltd
-

- Nintedanib
656247-17-5
- US $38.00-64.00 / mg
- 99.92%
- TargetMol Chemicals Inc.