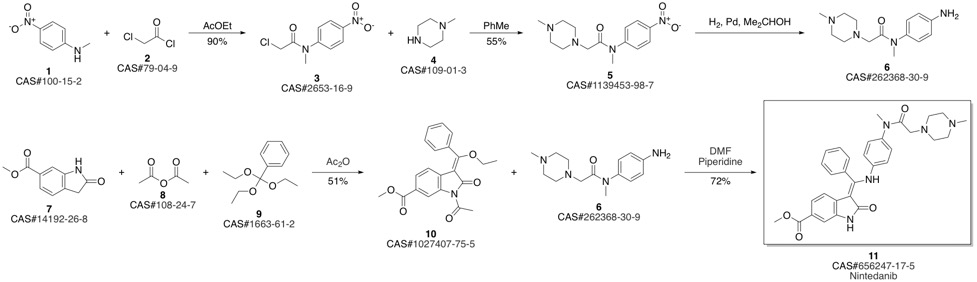
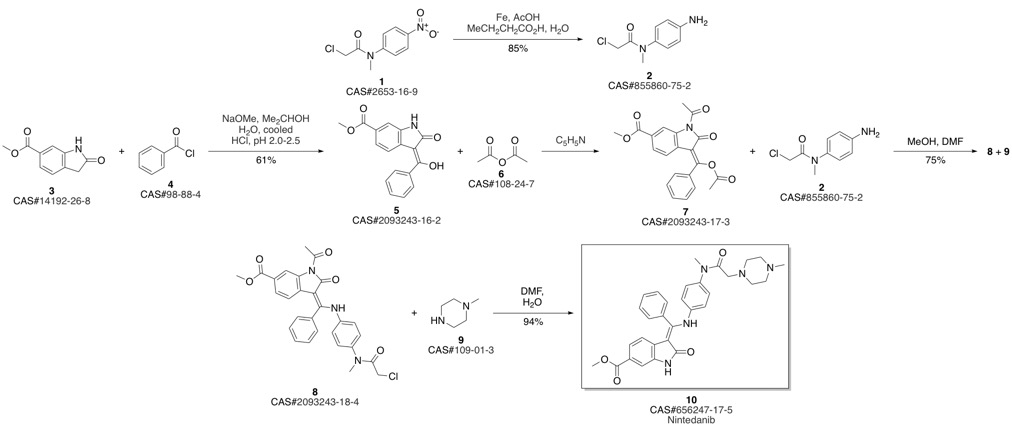
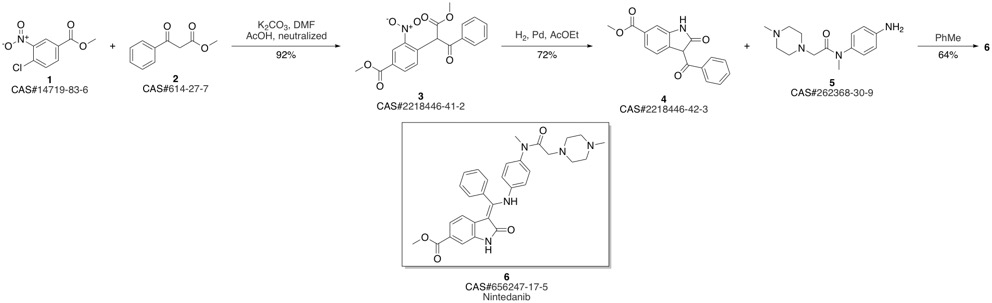
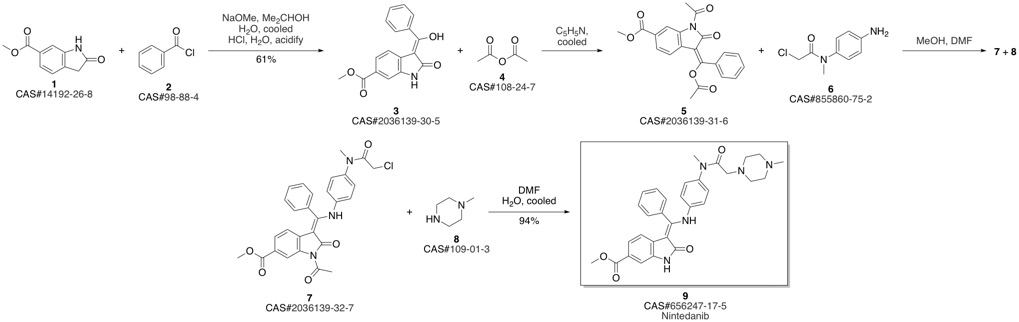
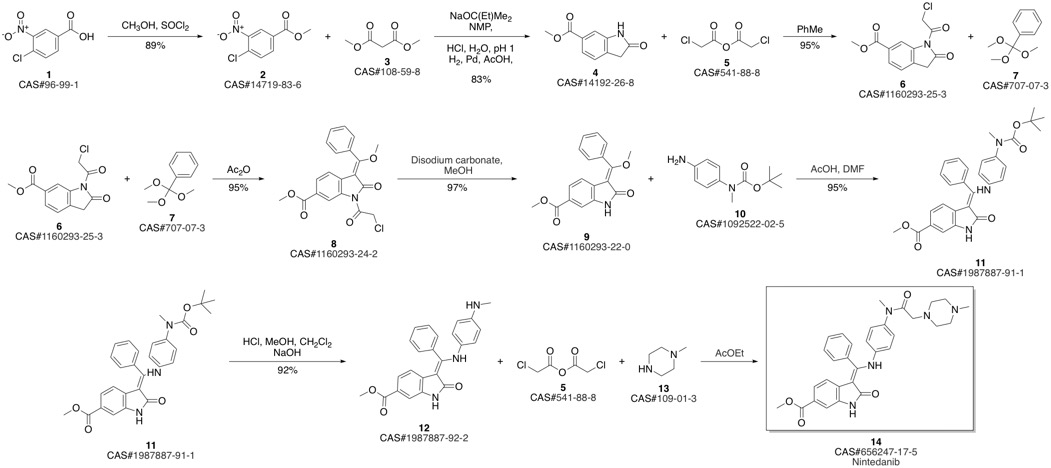
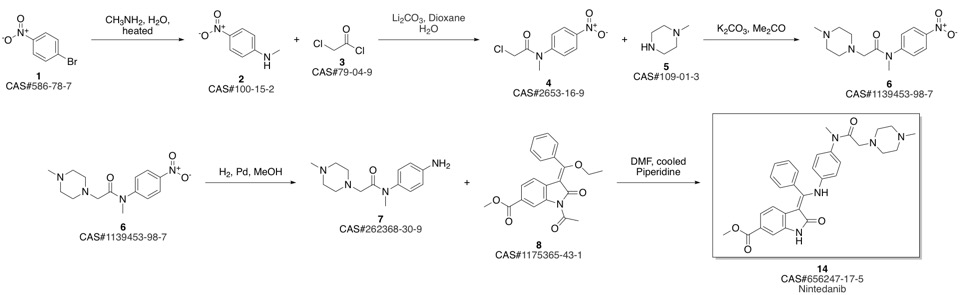
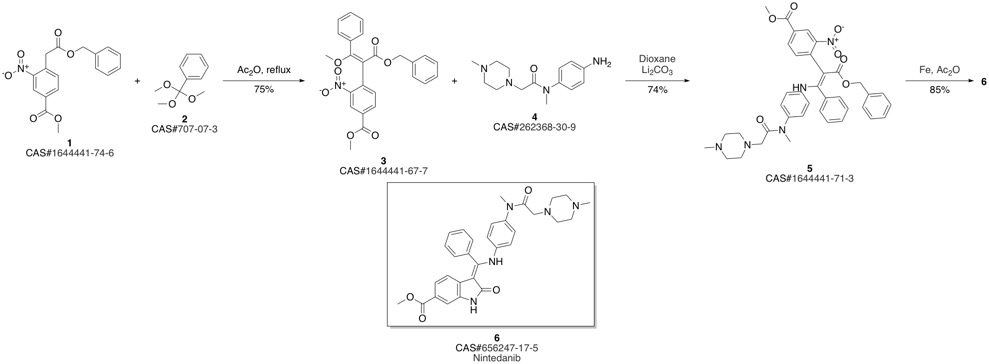
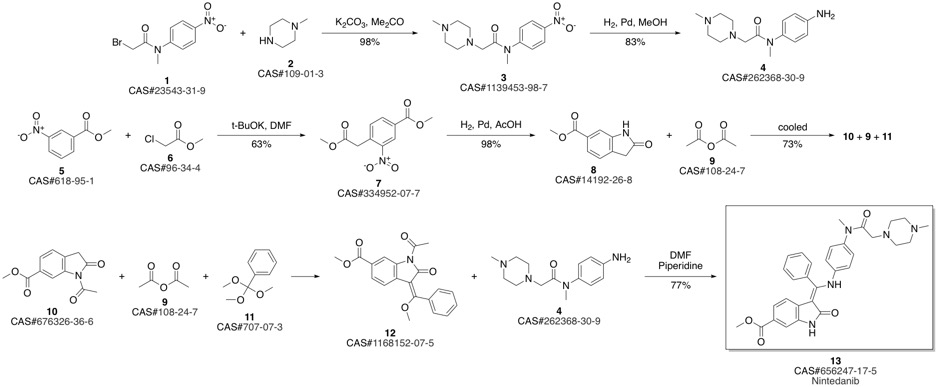
Nintedanib synthesis
- Product Name:Nintedanib
- CAS Number:656247-17-5
- Molecular formula:C31H33N5O4
- Molecular Weight:539.64
Roth, Gerald J.; Heckel, Armin; Colbatzky, Florian; Handschuh, Sandra; Kley, Jorg; Lehmann-Lintz, Thorsten; Lotz, Ralf; Tontsch-Grunt, Ulrike; Walter, Rainer; Hilberg, Frank. Design, Synthesis, and Evaluation of Indolinones as Triple Angiokinase Inhibitors and the Discovery of a Highly Specific 6-Methoxycarbonyl-Substituted Indolinone (BIBF 1120). Journal of Medicinal Chemistry. Volume 52. Issue 14. Pages 4466-4480. Journal. (2009).
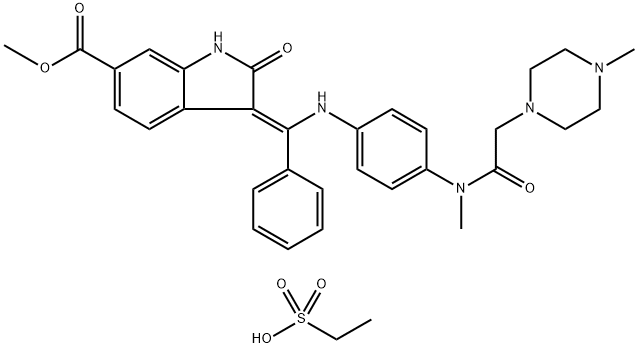
656247-18-6
302 suppliers
$7.00/100mg

656247-17-5
368 suppliers
$5.00/10mg
Yield:656247-17-5 98.7%
Reaction Conditions:
with sodium hydrogencarbonate in water;ethyl acetate at 40; for 1 h;
Steps:
21 Example 21
A nintedanib dilauryl sulfate salt was prepared by the following general procedure: a. 15 g of nintedanib esylate was added to a co-solvent of ethyl acetate/l0% aqueous NaHC03 (450 ml/l50 ml) (30V/10V) and stirred at 40°C for 1 hour; (0326) b. The organic layer of the reaction mixture of step (a) was separated and washed twice with 150 mL of purified water (10V x 2); (0327) c. The organic extracts of step (b) were combined and concentrated to obtain nintedanib free base as a yellow powder (12.3 g, 98.7% yield)
References:
HANDA PHARMACEUTICALS, LLC;LIU, Fang-Yu;SUNG, K.C.;YANG, Chin-Yao;LIN, Chi-Cheng;LIN, Yi-Hsin;QIAO, Li WO2019/241504, 2019, A1 Location in patent:Page/Page column 74-75
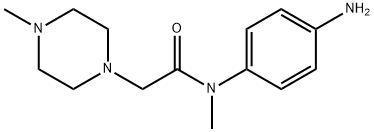
262368-30-9
255 suppliers
$5.00/250mg
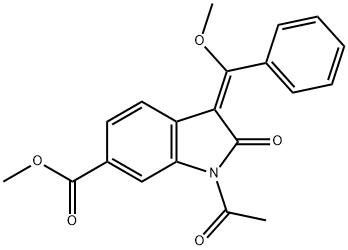
1168152-07-5
50 suppliers
inquiry

656247-17-5
368 suppliers
$5.00/10mg

262368-30-9
255 suppliers
$5.00/250mg
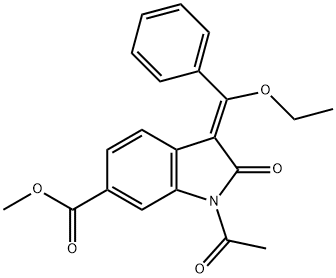
1027407-75-5
18 suppliers
inquiry

656247-17-5
368 suppliers
$5.00/10mg

262368-30-9
255 suppliers
$5.00/250mg
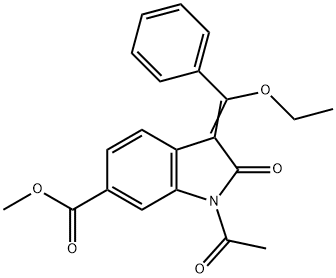
1175365-43-1
6 suppliers
inquiry

656247-17-5
368 suppliers
$5.00/10mg
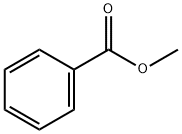
93-58-3
472 suppliers
$13.50/100 mL

262368-30-9
255 suppliers
$5.00/250mg
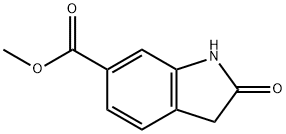
14192-26-8
372 suppliers
$10.00/250mg

656247-17-5
368 suppliers
$5.00/10mg