1-Acetyl-3-(ethoxyphenylmethylene)-2,3-dihydro-2-oxo-1H-indole-6-carboxylic acid methyl ester synthesis
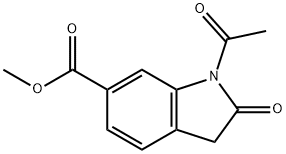
- Product Name:1-Acetyl-3-(ethoxyphenylmethylene)-2,3-dihydro-2-oxo-1H-indole-6-carboxylic acid methyl ester
- CAS Number:1175365-43-1
- Molecular formula:C21H19NO5
- Molecular Weight:365.38
Yield:1175365-43-1 85.2%
Reaction Conditions:
with acetic anhydrideReflux;
Steps:
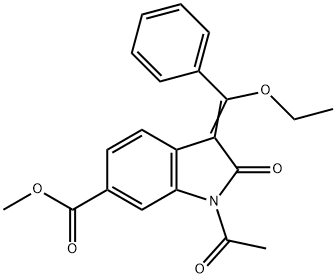
5 Example 5 Synthesis of the compound of formula (IV-b) (1-acetyl-3-(1-ethoxy-1-phenylmethylene)-6-methoxycarbonyl-2-oxyindole)
Suspend the compound of formula III (25.0g, 0.11mol) in acetic anhydride (175mL), then add triethyl orthobenzoate (72.0g, 0.32mol), heat to reflux with stirring, and react for 6-10 hours. The low boiling point mixture was removed by distillation, and TLC monitored the end of the reaction.Cool down to 2030 and filter.Rinse the filter cake with ethyl acetate (10ml).It was vacuum dried at 50°C to obtain 33.3 g of the compound of formula (IV-b) as a pale yellow to yellow solid, with a yield of 85.2%.
References:
CN111662223,2020,A Location in patent:Paragraph 0102-0107

108-24-7
5 suppliers
$14.00/250ML
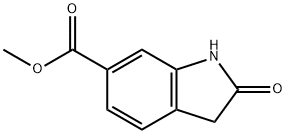
14192-26-8
371 suppliers
$10.00/250mg

1663-61-2
191 suppliers
$10.00/5g

1175365-43-1
6 suppliers
inquiry

14192-26-8
371 suppliers
$10.00/250mg

1175365-43-1
6 suppliers
inquiry