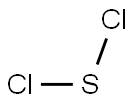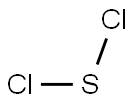Sulfur dichloride
- CAS No.
- 10545-99-0
- Chemical Name:
- Sulfur dichloride
- Synonyms
- SCl2;dichlorosulfoxide;hypochlorous thioanhydride;U.N. 1828;Dichloride(DDH);chlorinesulfide;Dichlorosulfane;Chlorine sulfide;Dichloro sulfide;sulfur dichlorde
- CBNumber:
- CB7413610
- Molecular Formula:
- Cl2S
Lewis structure

- Molecular Weight:
- 102.97
- MDL Number:
- MFCD00011445
- MOL File:
- 10545-99-0.mol
| Melting point | -122°C |
|---|---|
| Boiling point | 59.6°C (estimate) |
| Density | 1.355 g/mL at 25 °C |
| vapor density | 3.55 (vs air) |
| vapor pressure | 25.67 psi ( 55 °C) |
| solubility | reacts with H2O |
| form | red viscous liquid |
| color | red viscous liquid |
| CAS DataBase Reference | 10545-99-0(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8Q6684WQ9H |
| NIST Chemistry Reference | Sulfur dichloride(10545-99-0) |
| EPA Substance Registry System | Sulfur chloride (SCl2) (10545-99-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H335-H400 | |||||||||
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P273-P391-P501 | |||||||||
| Hazard Codes | T,N,C | |||||||||
| Risk Statements | 23/24/25-34-40-50-37-14-35 | |||||||||
| Safety Statements | 26-27-28-36/37/39-45-61 | |||||||||
| RIDADR | UN 3390 6.1/PG 1 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28121042 | |||||||||
| NFPA 704 |
|
Sulfur dichloride Chemical Properties,Uses,Production
Chemical Properties
Reddish-brown, fuming liquid; pungent chlorine odor. Decomposes in water and alcohol; soluble in benzene.
Uses
Chlorine carrier or chlorinating agent, rubber vulcanizing, vulcanized oils, purifying sugar juices, sulfur solvent, chloridizing agent in metallurgy, manufacture of organic chemicals and insecticides.
Hazard
Toxic by inhalation and ingestion, strong irritant to tissue.
Safety Profile
Poison irritant and corrosive to shin, eyes, and mucous membranes. Flammable when exposed to heat or flame. Reacts violently with Al, NH3, K, Na, acetone, dunethyl sulfoxide, water, oxidants, metals, hexafluoroisopropylidene amino lithium, and toluene. Reactive with water or steam. When heated to decomposition it emits very toxic fumes of SOx and Cl-.
Purification Methods
Distil sulfur chloride twice in the presence of a small amount of PCl3 through a 12in Vigreux column (p 11), the fraction boiling between 55-61o being redistilled (in the presence of PCl3), and the fraction distilling between 58-61o retained. (The PCl3 is added to inhibit the decomposition of SCl2 into S2Cl2 and Cl2). The SCl2 must be used as quickly as possible after distillation — within 1hour at room temperature. The sample contains 4% of S2Cl2. On long standing this reaches 16-18%. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 371-372 1963.] HARMFUL VAPOURS.
Sulfur dichloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of5
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32343 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23912 | 58 |
| Zhanhua Darong Chemical Technology Co., Ltd. | 0532-67773200 | sales@darongchem.com | China | 35 | 60 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 | cdhxsj@163.com | China | 13350 | 58 |
Related articles
- Why is SCl2 polar?
- The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atom....
- Dec 20,2023