Celecoxib
- CAS No.
- 169590-42-5
- Chemical Name:
- Celecoxib
- Synonyms
- CELEBREX;Celocoxib;Celecoxib (200 mg);4-[5-(4-Methylphenyl)-3-trifluoromethyl)-1H-pyrazol-yl]benzenesulfonamide Celecoxib;YM 177;ecoxib;CS-453;Celebra;Celecox;CS-1846
- CBNumber:
- CB7444681
- Molecular Formula:
- C17H14F3N3O2S
- Molecular Weight:
- 381.37
- MDL Number:
- MFCD00941298
- MOL File:
- 169590-42-5.mol
- MSDS File:
- SDS
| Melting point | 157-159°C |
|---|---|
| Boiling point | 529.0±60.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >20mg/mL |
| pka | 9.68±0.10(Predicted) |
| form | powder |
| color | white to off-white |
| Water Solubility | 7mg/L(25 ºC) |
| Merck | 14,1956 |
| BCS Class | 2 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChI | InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) |
| InChIKey | RZEKVGVHFLEQIL-UHFFFAOYSA-N |
| SMILES | C1(S(N)(=O)=O)=CC=C(N2C(C3=CC=C(C)C=C3)=CC(C(F)(F)F)=N2)C=C1 |
| CAS DataBase Reference | 169590-42-5(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | celecoxib |
| FDA UNII | JCX84Q7J1L |
| NCI Drug Dictionary | Celebrex |
| ATC code | L01XX33,M01AH01 |
| EPA Substance Registry System | Benzenesulfonamide, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]- (169590-42-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H360D-H373-H410 |
| Precautionary statements | P202-P260-P273-P280-P308+P313-P391 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-52-61-60 |
| Safety Statements | 22-24/25-28-37/39 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | DB2944937 |
| HazardClass | IRRITANT |
| HS Code | 29350090 |
Celecoxib price More Price(60)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML3031 | Celecoxib ≥98% (HPLC) | 169590-42-5 | 10MG | $94.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML3031 | Celecoxib ≥98% (HPLC) | 169590-42-5 | 50MG | $381 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR1683 | Celecoxib Pharmaceutical Secondary Standard; Certified Reference Material | 169590-42-5 | 1g | $111 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP1230 | Celecoxib British Pharmacopoeia (BP) Reference Standard | 169590-42-5 | 100MG | $275 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1098504 | Celecoxib United States Pharmacopeia (USP) Reference Standard | 169590-42-5 | 200mg | $436 | 2024-03-01 | Buy |
Celecoxib Chemical Properties,Uses,Production
Indications and Usage
Celecoxib and Rofecoxib are two currently used COX-2 inhibitors. Successfully developed by GD Searle & Pfizer Co. (U.S.,) released in 1999, trade name: Celebrex.
Celecoxib is a nonsteroidal, anti-inflammatory agent with significant analgesic and anti-inflammatory effects, causing the lowest incidence of upper gastrointestinal tract ulcers and other complications. Used clinically to treat acute and chronic osteoarthritis and rheumatoid arthritis, with an anti-inflammatory analgesic role, relieving the signs and symptoms of osteoarthritis and rheumatoid arthritis.
Application in Particular Diseases
In Osteoarthritis:
As a Cyclooxygenase-2 (COX-2) selective inhibitor, Celecoxib demonstrate analgesic benefits that are similar to traditional nonselective NSAIDs. Although COX-2 selective inhibition was designed to reduce NSAIDinduced gastropathy (e.g., ulcers, bleeding, perforation), concerns about adverse cardiovascular events (e.g., myocardial infarction, stroke) have led authorities to recommend their use only in selected patients who are at high risk for NSAID-related GI effects and low risk for cardiovascular toxicity.
Mechanisms of Action
Celecoxib has the anti-inflammatory and analgesic effect of NSAIDs. Because of its chemical structure, it can be combined with COX-2, selectively inhibiting COX-2. Its phenyl group binds with the hydrophobic channel of COX-2, and its hydrophilic sulfonamide forms a hydrogen chain with 513 arginine and 90 histidine in the COX-2 "side pocket.” It is also in close contact with arginine in the COX-2120 position and plays a role in inhibiting COX-2 from converting arachidonic acid to prostaglandins which are harmful to the human body. Due to subtle differences between the structures of COX-1 and COX-2, Celecoxib cannot enter the COX-1 molecule, nor inhibit its transformation of arachidonic acid into prostaglandins. Thus, it has good anti-inflammatory and analgesic effects, protects gastric mucosa, protects renal blood flow, regulates platelet aggregation, and resolves the gastric irritation problems of commonly used NSAIDs.
Pharmacokinetics
Celecoxib can be quickly absorbed after oral administration, with peak concentration at about 3h. The peak concentration of a single oral dose of 200 mg is 705 ng/ml. After absorption, up to 97% binds to plasma proteins; the drug is widely distributed, with apparent volume of distribution (455±166)L. Metabolized in vivo through P450(CYP)2C9 liver enzyme metabolism. Combined with carboxylic acid and glucuronide through biotransformation, about 2% of original amount excreted in urine and feces. Half-life about 1h.
Drug disposition and pharmacokinetics vary with race and age, amplifying total mild liver absorption damage (AUC.) When ingested with high fat peak plasma concentration effects are extended 1-2h, and AUC increases 10%-20%. When taken with aluminum and magnesium antacids peak concentration is reduced 37%, and AUC decreases 10%. No interaction with warfarin, ketoconazole, or methotrexate (MTX.) Clinically significant interactions with fluconazole and lithium, reducing Cmax 37% and AUC 10%。
Clinical Research
50 Clinical trials on more than 130,000 subjects in 23 countries have been conducted on Celecoxib. Regarding anti-inflammatory and analgesic effects towards osteoarthritis (OA) and rheumatoid arthritis (RA): subjects ages 18-93 had been diagnosed with OA or RA for at least three months, and the disease was in an active or relapsed state. Diagnoses were made according to the criteria of the American College of Rheumatology (ACR.) Visual analog scales (VAS) and American Pain Society (APS) test tables were used to evaluate joint pain, and the WOMAC and health assessment questionnaire (HAQ) impaired function indices were used to evaluate joint pain, stiffness, and function loss. Extensive clinical trials have shown that Celecoxib is effective against OA and RA joint pain.
Clinical trials on the efficacy of Celecoxib against OA have concluded: by VAS evaluation, 100 and 200 mg bid have the same effect on reducing signs and symptoms of OA as with 500 mg bid of naproxen, greater than with placebos, although the effects of 200 mg bid are not significantly greater than those with 100 mg bid. The effects on OA pain last 24-48h, equivalent to 500 mg bid of naproxen. Using the WOMAC (pain, joint stiffness, and impaired function) indices, 100 and 200 mg bid had the same effects as 500 mg bid of naproxen. 200 mg qd and 100 mg bid had similar effects on OA symptoms.
For RA: 100 and 200 mg bid significantly relieved RA symptoms in clinical trials, equivalent to 500 mg bid of naproxen. 100, 200, and 400 mg bid had similar effects on the number of RA patients suffering from joint swelling and pain/tenderness, all equivalent to 500 mg bid of naproxen.
Side Effects
Occasional gastrointestinal reactions. Small effects on renal function and platelets. Acute overdose can cause fatigue, drowsiness, nausea, vomiting, and upper abdominal pain, which can be relieved with treatment. Gastrointestinal bleeding is possible. In rare cases, hypertension, acute renal failure, respiratory depression, and comas may occur.
Warnings and Precautions
Anyone who is allergic to any ingredient in Celecoxib or who is known to be allergic to sulfa drugs should not take it.
It should be avoided in patients with asthma, urticaria, or acute rhinitis.
Lactating women should not take it.
May slow down metabolism and increase serum concentration in combination with leukotriene antagonist zafirlukast, antifungal agent fluconazole, and lipid-lowering statin drug fluvastatin.
Celecoxib can increase blood concentration of beta blockers, antidepressants and antipsychotic drugs, so care should be taken when using these drugs.
Description
Celecoxib is a nonsteroidal antiinflammatory drug (NSAID) first launched as Celebrex in the US for the treatment of symptoms in patients with rheumatoid arthritis (RA) and osteoarthritis (OA). Celecoxib belongs to a new class of 1, 5-diarylpyrazoles and can be synthesized by heat-promoted heterocyclization of a trifiuoro-l,3-dione with appropriate arylhydrazine. Celecoxib is a highly selective inhibitor of COX-2, the inducible form of cyclooxygenase expressed during inflammatory processes; it does not block the constitutive form COX-1, thus suppressing the gastric and intestinal toxicity of most non-selective NSAIDs. The potency ratio COX1/COX2 on purified human enzymes was about 400. In several in vivo models of acute and chronic inflammation, Celecoxib demonstrated potent antiinflammatory activity without affecting gastric or urinary prostaglandin PGE2. In several clinical studies performed with patients suffering from osteoarthritis or rheumatoid arthritis, Celecoxib was shown to be well tolerated and to relieve pain and inflammation more efficiently compared with other standard NSAIDs; the gastrointestinal safety profile was significantly better than that of other NSAIDs. Interestingly, Celecoxib was approved for another indication in patients with familial adenomatous polyposis (FAP). A six-month clinical trial demonstrated a 28% reduction in the number of colorectal polyps with Celecoxib, compared to a 5% reduction with placebo.
Chemical Properties
White to Pale Yellow Solid
Originator
Searle (US)
Uses
For relief and management of osteoarthritis (OA), rheumatoid arthritis (RA), juvenile rheumatoid arthritis (JRA), ankylosing spondylitis, acute pain, primary dysmenorrhea and oral adjunct to usual care for patients with familial adenomatous polyposis
Uses
expectorant, gastric stimulant, insecticide
Uses
A selective cyclooxygenase-2 (COX-2) inhibitor. Anti-inflammatory. Used in treatment of familial adenomatous polyposis
Definition
ChEBI: Celecoxib is a member of the class of pyrazoles that is 1H-pyrazole which is substituted at positions 1, 3 and 5 by 4-sulfamoylphenyl, trifluoromethyl and p-tolyl groups, respectively. A cyclooxygenase-2 inhibitor, it is used in the treatment of arthritis. It has a role as a cyclooxygenase 2 inhibitor, a geroprotector, a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is a member of toluenes, a sulfonamide, a member of pyrazoles and an organofluorine compound.
Indications
Celecoxib is indicated for the treatment of osteoarthritis and rheumatoid arthritis. Its use is contraindicated in individuals with hypersensitivity to sulfonamides or other NSAIDs. It should be used with caution in persons with hepatic disease. Interactions occur with other drugs that induce CYP2C9 (e.g. rifampin rifampin) or compete for metabolism by this enzyme (e.g. fluconazole, leflunomide). The most common adverse reactions to celecoxib are mild to moderate GI effects such as dyspepsia, diarrhea, and abdominal pain. Serious GI and renal effects have occurred rarely.
Manufacturing Process
4-(5-(4-Methylphenyl)-3-trifluoromethyl-N-pyrazol-1-yl)benzenesulfonamide To a solution of ethyl trifluoroacetate (1.90 ml, 16.0 mmol) in 7 ml of methyl tert-butyl ether was added 25% NaOMe (3.62 ml, 16.8 mmol). Next 4- chloroaceteophenone (2.08 ml, 16.0 mmol) in 2 ml of methyl tert-butyl ether was added. The mixture was stirred at room temperature overnight. To above solution was added 100 ml of 90% EtOH, followed by 4 N HCl (4.0 ml, 16 mmol) and 4-sulphonamidophenylhydrazine hydrochloride (3.58 g, 16 mmol). The mixture was heated to reflux for 3 hours. The mixture was concentrated. When 30 ml of water was added, a solid formed. The solid was filtered and washed with 20 ml of 60% EtOH to give 4.50 g of white solid. The filtrate was evaporated and taken up in ethyl acetate (100 ml), washed with saturated NaHCO3, and brine, dried over MgSO4, and concentrated. Heptane was added at boiling point of the mixture. After cooling down to 0°C, 1.01 g more product was obtained. The combined yield of the 4-(5-(4-methylphenyl)-3- trifluoromethyl-N-pyrazol-1-yl)benzenesulfonamide (Celecoxib) was 86%.
brand name
Celebrex (Searle).
Therapeutic Function
Antiinflammatory
General Description
Celecoxib (Celebrex) was the first selective COX-2 inhibitordrug introduced into the market in 1998 for use in thetreatment of RA, OA, acute pain, and menstrual pain. Thereal benefit is that it has caused fewer GI complicationswhen compared with other conventional NSAIDs. It hasalso been approved for reducing the number of adenomatouscolorectal polyps in familial adenomatous polyposis (FAP).Celecoxib is well absorbed and undergoes rapid oxidativemetabolism via CYP2C9 to give its inactive metabolites. Thus, a potential drug interaction exists betweencelecoxib and warfarin because the active isomer ofwarfarin is primarily degraded by CYP2C9.
Biological Activity
Selective cyclooxygenase-2 (COX-2) inhibitor (IC 50 values are 15 and 0.04 μ M for COX-1 and COX-2 respectively). Anti-inflammatory with shorter plasma half-life in vivo than SC 58121 (5-(4-Fluorophenyl)-1-[4-(methylsulfonyl)phenyl]-3-(trifluoromethyl)-1H-pyrazole ). Displays chemopreventive activity in in vivo tumor models.
Biochem/physiol Actions
Celecoxib is a non-steroidal, anti-inflammatory drug (NSAID) and a cyclooxygenase-2 (COX-2) selective inhibitor. Celecoxib is at least 10-20 times more selective for COX-2 over COX-1.
Pharmacokinetics
Celecoxib is well absorbed from the GI tract, with peak plasma concentrations generally being attained within 3 hours of administration. Peak plasma levels in geriatric patients may be increased, but dosage adjustments in elderly patients generally are not required unless the patient weighs less than 50 kg.
Clinical Use
Celecoxib is currently indicated for the relief of signs and symptoms of osteoarthritis and rheumatoid arthritis and to
reduce the number of adenomatous colorectal polyps in familial adenomatous polyposis as an adjunct to usual care.
Celecoxib is synthesized by condensing 4-methyl-acetophenone and ethyltrifluoroacetate with sodium methoxide and
the resulting butanedione derivative cyclized with 4-hydrazinophenylsulfonamide. It was the first NSAID to be
marketed as a selective COX-2 inhibitor.
Synthesis
Celecoxib is prepared by condensation of 4-methylacetophenone with ethyl trifluoroacetate to give 4,4,4-trifluoro-1-(4- methylphenyl)butane-1,3-dione, which is cyclized with 4-hydrazinophenylsulfonamide.
in vitro
celecoxib not only reduced the production of pge2 but also inhibited the downstream effects of pge2. celecoxib blocked migration and invasion of a549 cells increased by pge2 in the wound healing and transwell assays. additionally, celecoxib reduced mmp9 mrna expression which was increased by pge2. moreover, celecoxib enhanced e-cadherin mrna expression which was inhibited by pge2 [2].
in vivo
celecoxib inhibited the increase in metastases of a549 cells and significantly reduced the increase in pge2 plasma level in mice receiving unilateral pneumonectomy [2].
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones; concentration reduced by
rifampicin
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced
Antiepileptics: possibly increased phenytoin
concentration.
Antifungals: if used with fluconazole, halve the dose
of celecoxib.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: possibly increased risk of bleeding
Tacrolimus: increased risk of nephrotoxicity.
Metabolism
Celecoxib is excreted in the urine and feces primarily as inactive metabolites, with less than 3% of an administered dose being excreted as unchanged drug. Metabolism occurs primarily in the liver by CYP2C9 and involves hydroxylation of the 4-methyl group to the primary alcohol, which is subsequently oxidized to its corresponding carboxylic acid, the major metabolite (73% of the administered dose). The carboxylic acid is conjugated, to a slight extent, with glucuronic acid to form the corresponding glucuronide. None of the isolated metabolites have been shown to exhibit pharmacological activity as inhibitors of either COX-1 or COX-2. Celecoxib also inhibits CYP2D6; thus, the potential of celecoxib to alter the pharmacokinetic profiles of other drugs inhibited by this isoenzyme exists. Celecoxib, however, does not appear to inhibit other CYP isoforms, such as CYP2C19 or CYP3A4. Other drug interactions related to the metabolic profile of celecoxib have been noted, particularly with other drugs that inhibit CYP2C.
storage
Store at RT
References
1) Penning . (1997), Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib); J. Med. Chem., 40 1347 2) DeWitt et al. (1999), Cox-2-selective inhibitors: the new super aspirins; Mol. Pharmacol., 55 625 3) Harris et al. (2000), Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor; Cancer Res., 60 2101 4) Koki and Masferrer et al. (2002), Celecoxib: a specific COX-2 inhibitor with anticancer properties; Cancer Control, 9 28
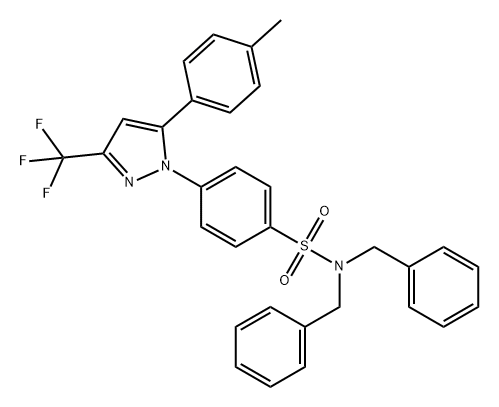
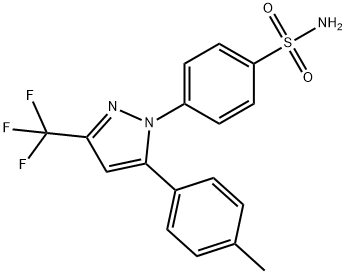
Celecoxib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@sdzhonghuimaterial.com | China | 983 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659 +86-13153181156 | sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 283 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 | xinshengkeji@xsmaterial.com | China | 1085 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 615 | 58 |
Related articles
- Celecoxib: A Detailed Exploration of Its Chemistry, Applications, and Storage
- Celecoxib has emerged as a widely prescribed medication due to its targeted mechanism of action and its reduced side effects c....
- Sep 24,2024
View Lastest Price from Celecoxib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | Celecoxib
169590-42-5
|
US $6.00 / kg | 1kg | More than 99% | 2000KG/Month | Hebei Longbang Technology Co., Ltd | |
 |
2024-11-28 | Celecoxib
169590-42-5
|
US $0.00-0.00 / Kg/Bag | 1Kg/Bag | 99% min / USP/ EP / GMP application / PMDA / DMF | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-28 | Celecoxib
169590-42-5
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd |
169590-42-5(Celecoxib)Related Search:
1of4





