Argatroban
- CAS No.
- 74863-84-6
- Chemical Name:
- Argatroban
- Synonyms
- Argatraban;(2R,4R)-4-Methyl-1-[N-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]-L-arginyl]-2-piperidinecarboxylic acid;MQP;MQPA;MD-805;ARG025;OM 805;DK-7419;Slonnon;MCI-9038
- CBNumber:
- CB7738830
- Molecular Formula:
- C23H36N6O5S
- Molecular Weight:
- 508.64
- MDL Number:
- MFCD23102419
- MOL File:
- 74863-84-6.mol
| Melting point | 188-1890C |
|---|---|
| Boiling point | 777.2±70.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Soluble in DMSO (up to 50 mg/ml). |
| pka | 10.44±0.40(Predicted) |
| form | White to off-white powder. |
| color | White |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChIKey | KXNPVXPOPUZYGB-IOVMHBDKSA-N |
| SMILES | N1(C(=O)[C@@H](NS(C2C3=C(C=CC=2)CC(C)CN3)(=O)=O)CCCNC(N)=N)CC[C@@H](C)C[C@@H]1C(O)=O |
| CAS DataBase Reference | 74863-84-6(CAS DataBase Reference) |
| FDA UNII | OCY3U280Y3 |
| ATC code | B01AE03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H311-H331 | |||||||||
| Precautionary statements | P261-P264-P270-P271-P280-P302+P352-P304+P340-P310-P330-P361-P403+P233-P405-P501 | |||||||||
| Hazardous Substances Data | 74863-84-6(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Argatroban price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 19639 | Argatroban ≥95% | 74863-84-6 | 5mg | $37 | 2024-03-01 | Buy |
| Cayman Chemical | 19639 | Argatroban ≥95% | 74863-84-6 | 10mg | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 19639 | Argatroban ≥95% | 74863-84-6 | 25mg | $142 | 2024-03-01 | Buy |
| Cayman Chemical | 19639 | Argatroban | 74863-84-6 | 50mg | $266 | 2024-03-01 | Buy |
| Tocris | 1637 | Argatroban ≥99%(HPLC) | 74863-84-6 | 50 | $770 | 2021-12-16 | Buy |
Argatroban Chemical Properties,Uses,Production
Description
Argatroban is a new synthetic antithrombotic agent useful in maintenance anticoagulation them ischemic stroke and disseminated intravascular coagulation. In patients on hemodialysis, argatroban is su enor to heparin, generating a more stable antithrombin effect. Other potential uses include progressing ischemic stroke and disseminated intravascular coagulation.
Description
Argatroban is a reversible inhibitor of thrombin (IC50s = 0.01 and 0.09 μM for the free and clot-bound forms, respectively). It is selective for thrombin over factor Xa, trypsin, plasmin, and tissue plasminogen activator (Kis = 0.0045, 53, 0.19, 25.7, and 87.7 μM, respectively). It inhibits thrombin-induced platelet aggregation in washed isolated guinea pig platelets with an IC50 value of 0.077 μM. Argatroban (3.2 mg/kg, s.c.) decreases thrombus formation in a guinea pig model of iron chloride-induced arterial thrombosis. Formulations containing argatroban have been used in the prevention or treatment of thrombosis in patients with heparin-induced thrombocytopenia.
Chemical Properties
White to Off-White Crystalline Solid
Originator
Mitsubishi Kasei; Daiichi (Japan)
Uses
anticoagulant;direct thrombine inhibitor
Uses
(2S,4S)-1-(2R)-Argatroban is an isomeric impurity of Argatroban (74863-84-6 #CAS), which is a synthetic thrombin inhibitor. Antithrombotic.
Definition
ChEBI: (2R,4R)-1-[(2S)-5-(diaminomethylideneamino)-2-[(3-methyl-1,2,3,4-tetrahydroquinolin-8-yl)sulfonylamino]-1-oxopentyl]-4-methyl-2-piperidinecarboxylic acid is a peptide.
Manufacturing Process
To a stirred solution of 28.3 g of NG-nitro-N2-(tert-butoxycarbonyl)-L-arginine
in 450 ml of dry tetrahydrofuran were added in turn 9.0 g of triethylamine
and 12.2 g of isobutyl chloroformate while keeping the temperature at -20°C.
After 10 minutes, to this was added 15.2 g of ethyl 4-methyl-2-
piperidinecarboxylate and the mixture was stirred for 10 minutes at -20°C. At
the end of this period, the reaction mixture was warmed to room
temperature. The solvent was evaporated and the residue taken up in 400 ml
of ethyl acetate, and washed successively with 200 ml of water, 100 ml of 5%
sodium bicarbonate solution, 100 ml of 10% citric acid solution and 200 ml of
water. The ethyl acetate solution was dried over anhydrous sodium sulfate.
The solution was evaporated to give 31.5 g (75 %) of ethyl 1-[NG-nitro-N2-
(tert-butoxycarbonyl)-L-arginyl]-4-methyl-2-piperidinecarboxylate in the form
of a syrup.
To a stirred solution of 30 g of ethyl 1-[NG-nitro-N2-(tert-butoxycarbonyl)-Larginyl]-
4-methyl-2-piperidinecarboxylate in 50 ml of ethyl acetate was added
80 ml of 10% dry HCl-ethyl acetate at 0°C. After 3 hours, to this solution was
added 200 ml of dry ethyl ether to precipitate a viscous oily product. This was
filtered and washed with dry ethyl ether to give ethyl 1-[NG-nitro-L-arginyl]-4-
methyl-2-piperidinecarboxylate hydrochloride as an amorphous solid.
To a stirred solution of ethyl 1-(NG-nitro-L-arginyl)-4-methyl-2-
piperidinecarboxylate hydrochloride in 200 ml of chloroform were added in
turn 18.5 g of triethylamine, and 14.7 g of 3-methyl-8-quinolinesulfonyl
chloride at 5°C, and stirring was continued for 3 hours at room temperature.
At the end of this period, the solution was washed twice with 50 ml of water.
The chloroform solution was dried over anhydrous sodium sulfate. Upon
evaporation of the solvent, the residue was chromatographed on 50 g of silica
gel packed in chloroform, washed with chloroform and eluted with 3%
methanol-chloroform. The fraction eluted from 3% methanol-chloroform was
evaporated to give 32.1 g (91%) of ethyl 1-[NG-nitro-N2-(3-methyl-8-
quinolinesulfonyl)-L-arginyl]-4-methyl-2-piperidinecarboxylate in the form of
an amorphous solid.
A solution of 30 g the above product in 100 ml of ethanol and 100 ml of 1 N
sodium hydroxide solution was stirred for 24 hrs at room temperature. At the
end of this period, the solution was neutralized with 1 N hydrochloric acid and
then concentrated to 70 ml. The solution was adjusted to pH=11 with 1 N
sodium hydroxide solution, washed three times with 100 ml of ethyl acetate,
acidified with 1 N hydrochloric acid and then extracted three times with 100
ml of chloroform. The combined chloroform solution was dried over anhydrous
sodium sulfate and evaporated to give 28.0 g (97%) of 1-[NG-nitro-N2-(3-
methyl-8-quinolinesulfonyl)-L-arginyl]-4-methyl-2-piperidinecarboxylic acid as
an amorphous solid. IR (KBr): 3,300, 1,720, 1,630 cm-1.
To a solution of 3.00 g of 1-[NG-nitro-N2-(3-methyl-8-quinolinesulfonyl)-Larginyl]-
4-methyl-2-piperidinecarboxylic acid in 50 ml of ethanol was added
0.5 g of palladium black and then the mixture was shaken under 10 kg/cm2
H2 pressure at 100°C for 8 hrs. At the end of this period, the ethanol solution
was filtered to remove the catalyst and evaporated to give 2.50 g (90%) of 1-
[N2-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl]-4-methyl-2-
piperidinecarboxylic acid as an amorphous solid. IR (KBr): 3,400, 1,620,
1,460, 1,380 cm-1.
brand name
Novastan (Mitsubishi Chemical Corporation, Japan);Slonnon.
Therapeutic Function
Anticoagulant
Pharmacokinetics
Argatroban is administered subcutaneously because of the low lipophilicity of the drug. The drug is bound to plasma protein and is metabolized via CYP3A4/5 to the aromatized metabolite and the two hydroxylated metabolites. The M-1 metabolite retains 20 to 30% of the antithrombotic activity. Coadministration of argatroban with inhibitors of CYP3A4 does not appear to produce clinically significant effects. Argatroban is eliminated via biliary secretion into the feces.
Clinical Use
Argatroban has been approved for the prophylaxis and treatment of thrombosis in patients with HIT (79). Argatroban is a peptidomimetic that binds selectively to the catalytic site of thrombin as a univalent competitive DTI. Argatroban is available as a mixture of 21-R and 21-S diastereomers (64:36), with the S-isomer approximately twice as potent as the R-isomer. The drug is a reversible inhibitor of both free thrombin as well as clot-bound thrombin.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of haemorrhage with IV
diclofenac and ketorolac - avoid.
Antiplatelets and anticoagulants: increased risk of
bleeding complications.
Heparin: avoid concomitant administration.
Urokinase: may increase the risk of bleeding.
Thrombolytics: may increase risk of bleeding
complications; enhance effect of argatroban.
Metabolism
The metabolism of argatroban has not yet been fully
characterised. The metabolites identified (M-1, M-2, and
M-3) are formed by hydroxylation and aromatisation
of the 3-methyltetrahydroquinoline ring in the liver.
The main metabolite (M1) exerts 40-fold weaker
antithrombin effect than argatroban. Metabolites M-1,
M-2 and M-3 were detected in the urine, and M-1 was
detected in plasma and faeces.
Argatroban is excreted mainly in the faeces, presumably
through biliary secretion. Following intravenous infusion of [14C]-argatroban 21.8±5.8% of the dose was excreted
in urine and 65.4±7.1% in the faeces.
storage
Store at +4°C
References
1) Kikumoto?et al. (1984),?Selective inhibition of thrombin by (2R,4R)-4-methyl-1-[N2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]-l-arginyl)]-2-piperidinecarboxylic acid; Biochemistry,?23?85 2) Jang?et al. (1990),?Prevention of platelet-rich arterial thrombosis by selective thrombin inhibition; Circulation,?81?219 3) Sugawara?et al. (2009),?Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats; Stroke,?40?1530 4) Schulze?et al. (2008),?The thrombin inhibitor Argatroban reduces breast cancer malignancy and metastasis via osteopontin-dependent and osteopontin-independent mechanisms; Breast Cancer Res. Treat.,?112?243

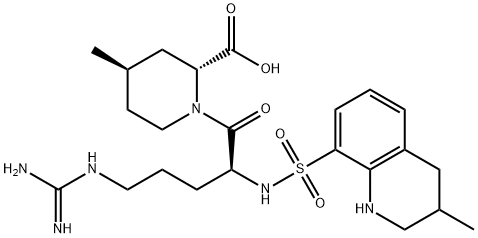
Argatroban Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15350 | 58 |
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 | yuxin@yuxchem.com | China | 167 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21631 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29863 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
View Lastest Price from Argatroban manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-01-13 | Argatroban
74863-84-6
|
US $0.00 / g | 1g | More Than 99% | 100kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | |
 |
2024-11-19 | Argatroban
74863-84-6
|
US $33.00-105.00 / mg | 99.77% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-19 | Argatroban
74863-84-6
|
US $33.00-105.00 / mg | 99.77% | 10g | TargetMol Chemicals Inc. |
-

- Argatroban
74863-84-6
- US $0.00 / g
- More Than 99%
- BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
-

- Argatroban
74863-84-6
- US $33.00-105.00 / mg
- 99.77%
- TargetMol Chemicals Inc.
-

- Argatroban
74863-84-6
- US $33.00-105.00 / mg
- 99.77%
- TargetMol Chemicals Inc.





