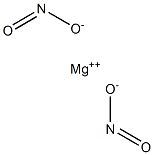
Bisnitrous acid magnesium salt
- CAS No.
- Chemical Name:
- Bisnitrous acid magnesium salt
- Synonyms
- Magnesium nitrite;Bisnitrous acid magnesium salt
- CBNumber:
- CB82137775
- Molecular Formula:
- MgN2O4
- Molecular Weight:
- 116.316
- MDL Number:
- MOL File:
- Mol file
| FDA UNII | 05MVC8T5JN |
|---|
Bisnitrous acid magnesium salt Chemical Properties,Uses,Production
Uses
The instability of magnesium nitrite, even when dissolved in water, has not engendered any usage in industry.
Preparation
Magnesium nitrite has the formula of Mg(NO2)2 and
the molecular weight of 116.3164 g/mol. It can be made
by the usual double decomposition reaction of sodium
nitrite and CaCl2:
2NaNO2 (aq) + MgCl2?Mg(NO2)2 (aq) + 4NaCl (aq)
It forms a trihydrate having a molecular weight of
170.3626 g/mol. Magnesium nitrite is soluble in water
and the solution must be evaporated at very low temperatures
to obtain crystals. The solution is stable and is
slightly hydrolyzed in the cold but at 60° and above it
evolves oxides of nitrogen. Its CAS number is 15070-34-5 and it occurs as white, hydroscopic crystals that
must be protected from moisture to prevent decomposition.
The trihydrate, when heated, forms the monohydrate
at 100 °C and then decomposes to NO + NO2
and MgO at >130 °C. It is soluble in alcohol.
Bisnitrous acid magnesium salt Preparation Products And Raw materials
Raw materials
Preparation Products
(Bisnitrous acid magnesium salt)Related Search:
1of4