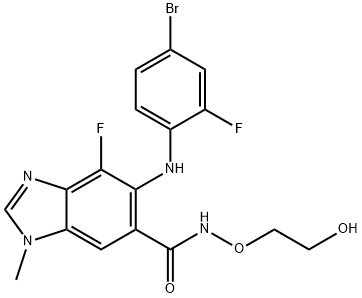
Binimetinib
- CAS No.
- 606143-89-9
- Chemical Name:
- Binimetinib
- Synonyms
- MEK162;ARRY-162;ARRY-438162;CS-394;ARRY 162;Binimetinib;MEK162, ARRY-162;Valine Impurity 34;MEK162(Binimetinib);MEK162 (ARRY-438162)
- CBNumber:
- CB82604200
- Molecular Formula:
- C17H15BrF2N4O3
- Molecular Weight:
- 441.23
- MDL Number:
- MFCD22124525
- MOL File:
- 606143-89-9.mol
| Melting point | >203oC (dec.) |
|---|---|
| Density | 1.67 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml) |
| pka | 14.20±0.10(Predicted) |
| form | solid |
| color | White |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| NCI Dictionary of Cancer Terms | binimetinib |
| FDA UNII | 181R97MR71 |
| NCI Drug Dictionary | binimetinib |
| ATC code | L01EE03 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H360-H362 |
| Precautionary statements | P201-P260-P263-P264-P270-P308+P313 |
| HS Code | 2933998090 |
Binimetinib price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML3385 | Binimetinib ≥98% (HPLC) | 606143-89-9 | 10MG | $50.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML3385 | Binimetinib ≥98% (HPLC) | 606143-89-9 | 50MG | $163 | 2024-03-01 | Buy |
| Cayman Chemical | 16996 | Binimetinib ≥98% | 606143-89-9 | 5mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 16996 | Binimetinib ≥98% | 606143-89-9 | 10mg | $107 | 2024-03-01 | Buy |
| Cayman Chemical | 16996 | Binimetinib ≥98% | 606143-89-9 | 50mg | $437 | 2024-03-01 | Buy |
Binimetinib Chemical Properties,Uses,Production
Kinase inhibitor
Binimetinib, also known as Mektovi, is a potent and selective oral mitogen-activated protein kinase 1/2 (MEK 1/2) inhibitor with potential antineoplastic activity.
Binimetinib, noncompetitive with ATP, binds to and inhibits the activity of MEK1/2. Inhibition of MEK1/2 prevents the activation of MEK1/2 dependent effector proteins and transcription factors, which may result in the inhibition of growth factor-mediated cell signaling. This may eventually lead to an inhibition of tumor cell proliferation and an inhibition in production of various inflammatory cytokines including interleukin-1, -6 and tumor necrosis factor.
Mechanism of Action
Binimetinib is a reversible inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, binimetinib inhibited extracellular signal-related kinase (ERK) phosphorylation in cellfree assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant human melanoma cell lines. Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models.
Pharmacokinetics
The primary metabolic pathway is glucuronidation with UGT1A1 contributing up to 61% of the binimetinib metabolism. Other pathways of binimetinib metabolism include N-dealkylation, amide hydrolysis, and loss of ethane-diol from the side chain. The active metabolite M3 produced by CYP1A2 and CYP2C19 represents 8.6% of the binimetinib exposure. Following a single oral dose of 45 mg radiolabeled binimetinib, approximately 60% of the circulating radioactivity AUC in plasma was attributable to binimetinib.
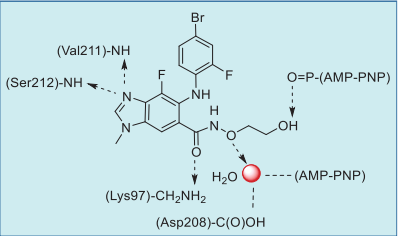
Binding Mode
As shown in the co-crystal structure of
binimetinib in complex with BRAF–MEK1 kinases
and AMP–PNP (Fig. 1), the imine nitrogen of the
benzo[d]imidazole core hydrogen bonds to both the
amide NH of Ser212 and amide NH of Val211, and
the amide oxygen also forms a hydrogen bond with
the primary amine of Lys97. In addition, the terminal
hydroxyl group hydrogen bonds to the α-phosphate
oxygen of AMP–PNP. Also, the carboxamide side
chain oxygen interacts indirectly with the carboxylic
acid of Asp208 and AMP–PNP via a water molecule
(Fig. 2).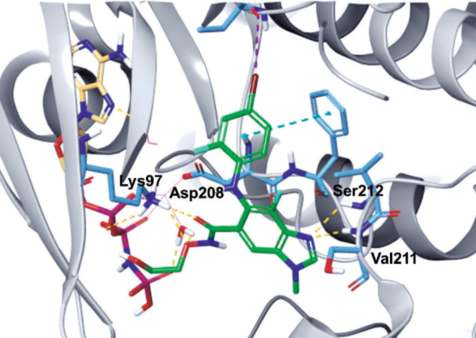
Description
Binimetinib (606143-89-9) is a potent (IC50?= 12 nM) and selective allosteric inhibitor of MEK1/2.1,2?Recently approved by the FDA for treatment of melanoma in combination with Encorafenib. Binimetinib has had limited success as monotherapy but has shown promise in combination with other chemotherapeutic agents.3-5
Uses
MEK 162 is a MEK1/2 inhibitor allowing it to be a effective anti-cancer medication.
Definition
ChEBI: Binimetinib is a member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-fluorophenyl)nitrilo, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectively. It is a MEK1 and MEK2 inhibitor (IC50= 12 nM). Approved by the FDA for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation in combination with encorafenib. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of benzimidazoles, a member of bromobenzenes, a member of monofluorobenzenes, a hydroxamic acid ester and a secondary amino compound.
brand name
Mektovi
General Description
Class: dual threonine/tyrosine kinase; Treatment: melanoma with BRAF mutations; Other name: ARRY-162; Oral bioavailability = 50%; Elimination half-life = 3.5 h; Protein binding = 97%
Pharmacokinetics
After oral administration, binimetinib is absorbed rapidly, with a median tmax of 1.48 h. Binimetinib is 50% orally bioavailable and exhibits a short elimination half-life of 3.5 h. Consequently, it requires twice-daily dosing regimen. Binimetinib undergoes UGT1A1-mediated glucuronidation, which contributes up to 61% of the overall metabolism. Other metabolic pathways include N-dealkylation, amide hydrolysis, and loss of ethanediol from the side chain.
target
Primary target: MEK1/2
References
1) Lee?et al.?(2010),?Preclinical development of ARRY-162, a potent and selective MEK1/2 inhibitor;?Cancer Res.?70?2515 2) Winski?et al.?(2010),?MEK162 (ARRY-162), a novel MEK ? inhibitor, inhibits tumor growth regardless of KRAS/RAF pathway mutations;?EJC Supplements?8?56 3) Lee?et al.?(2016),?Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models;?Oncotarget?7?39595 4) Gong?et al.?(2017),?MEK162 Enhances Antitumor Activity of 5-Fluorouracil and Trifluridine in KRAS-mutated Human Colorectal Cancer Cell Lines;?Anticancer Res.?37?2831 5) Van Cutsem?et al.?(2019),?Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From Phase III BEACON Colorectal Cancer study;?J. Clin. Oncol.?180?2459
Binimetinib Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 485 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29860 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 965 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 8497 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32159 | 58 |
Related articles
- The synthesis method of Binimetinib
- Binimetinib is a non-ATP competitive mitogen-activated protein kinase 1/2 (MEK1/2) inhibitor discovered by Array BioPharma.
- Dec 27,2023
- What is Binimetinib?
- Binimetinib is a kinase inhibitor. The chemical name is 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2hydroxyethoxy)- 1-methy....
- Feb 21,2020
View Lastest Price from Binimetinib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-01-13 | Binimetinib
606143-89-9
|
US $0.00 / g | 1g | More Than 99% | 100kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | |
 |
2024-11-26 | Binimetinib
606143-89-9
|
US $0.00-0.00 / kg | 1kg | 99%, Single impurity<0.1 | 1 ton | Nanjing Fred Technology Co., Ltd | |
 |
2024-11-19 | Binimetinib
606143-89-9
|
US $35.00-68.00 / mg | 99% | 10g | TargetMol Chemicals Inc. |
-

- Binimetinib
606143-89-9
- US $0.00 / g
- More Than 99%
- BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
-

- Binimetinib
606143-89-9
- US $0.00-0.00 / kg
- 99%, Single impurity<0.1
- Nanjing Fred Technology Co., Ltd
-

- Binimetinib
606143-89-9
- US $35.00-68.00 / mg
- 99%
- TargetMol Chemicals Inc.