Ebastine
- CAS No.
- 90729-43-4
- Chemical Name:
- Ebastine
- Synonyms
- EBASTIN;EBASTEL;LAS-90;Ebasine;Estivan;EVASTEL;KESTINE;ebastina;RP 64305;EBASTINE
- CBNumber:
- CB8271800
- Molecular Formula:
- C32H39NO2
- Molecular Weight:
- 469.66
- MDL Number:
- MFCD00865661
- MOL File:
- 90729-43-4.mol
- MSDS File:
- SDS
| Melting point | 80-82°C |
|---|---|
| Boiling point | 596.3±50.0 °C(Predicted) |
| Density | 1.09±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in chloroform, methanol. |
| pka | 8.19±0.10(Predicted) |
| form | solid |
| color | white |
| Merck | 14,3484 |
| CAS DataBase Reference | 90729-43-4(CAS DataBase Reference) |
| FDA UNII | TQD7Q784P1 |
| ATC code | R06AX22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H303 |
| Precautionary statements | P270-P301+P312-P403-P501c |
| WGK Germany | 3 |
| RTECS | EL8140000 |
| HS Code | 2933.39.9200 |
Ebastine price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | E9531 | Ebastine ≥98% (HPLC), solid | 90729-43-4 | 10mg | $257 | 2024-03-01 | Buy |
| Sigma-Aldrich | E9531 | Ebastine ≥98% (HPLC), solid | 90729-43-4 | 50mg | $853 | 2024-03-01 | Buy |
| TCI Chemical | E0925 | Ebastine >98.0%(HPLC)(T) | 90729-43-4 | 1g | $144 | 2024-03-01 | Buy |
| TCI Chemical | E0925 | Ebastine >98.0%(HPLC)(T) | 90729-43-4 | 5g | $494 | 2024-03-01 | Buy |
| Alfa Aesar | J63088 | Ebastine | 90729-43-4 | 1g | $146.65 | 2024-03-01 | Buy |
Ebastine Chemical Properties,Uses,Production
Description
Ebastine belongs to an effective second generation histamine H1 receptor antagonist. One important property of it is that it doesn’t penetrate the blood brain barrier. Therefore, it is capable of blocking the H1 receptor in peripheral tissue without having some specific side effects such as sedation and drowsiness. Ebastine is mainly applied for the treatment of allergic rhinitis, pruritus as well as acting as an alternative drug in Decongestant. Upon entering into the human body, it can subject to the action of hepatic cytochrome P450 3A4 to be converted to an active carboxylic acid metabolite, carebastine. The later one is the major active form in vivo. Under certain range, it can inhibit T cell proliferation and the production of Th2-type pro-inflammatory cytokines through macrophages. Ebastine generally has a high safety property without causing cognitive/psychomotor impairment and sedation, like placebo. However, it can cause side effects such as inflammation of the air-cavities around nose, sore throat, indigestion, nausea, headache, and abdominal pain.
References
https://pubchem.ncbi.nlm.nih.gov/compound/3191#section=Top
http://www.tabletwise.com/medicine/ebastine/side-effects
http://www.druginfosys.com/drug.aspx?drugcode=897&type=1
Description
Ebastine is a new once-daily histamine Hl-receptor antagonist with no sedative effects or autonomic impairment at therapeutic doses. It is reported to be effective in the treatment of hay fever, perennial rhinitis, and uticaria. Ebastine's antihistamine activity is attributed to its carboxylic acid metabolite carebastine.
Chemical Properties
White to Off-White Powder
Originator
Almirall (Spain)
Uses
A nonsedating type histamine H1-receptor antagonist. Antihistaminic
Uses
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic rhinitis and chronic idiopathic urticaria. It is available in 10 and 20 mg tablets and as fast-dissolving tablets, as well as in pediatric syrup. It has a recommended flexible daily dose of 10 or 20 mg, depending on disease severity.
Definition
ChEBI: Ebastine is an organic molecular entity.
Manufacturing Process
(a) A mixture of 4-hydroxypiperidine (40.4 g; 0.4 moles), p-tert-butyl-ω-
chlorobutyrophenone (105 g, 0.44 moles), sodium bicarbonate (67.2 g; 0.8
moles) and a crystal of potassium iodide in methyl isobutyl ketone (1 liter)
was boiled under reflux for 24 hours. After cooling, the reaction mixture waswashed with water, dried (Na2SO4) and the solvent removed in vacuum. The
residue was salified with the stoichiometric amount of fumaric acid in a
mixture of acetone and ethanol to give 1-[3-(4-tert-butylbenzoyl)propyl]-4-
hydroxypiperidine fumarate (148 g), melting point 163-165°C. This compound
was converted into the free base, and 1-[3-(4-tert-butylbenzoyl)propyl]-4-
hydroxypiperidine was obtained and recrystallized from a mixture of diethyl
ether and petroleum ether (boiling point 50-70°C). 102 g were obtained (yield
84%), melting point 63-65°C.
(b) A mixture of 1-[3-(tert-butylbenzoyl)propyl]-4-hydroxypiperidine (60.68 g;
0.2 moles) and sodium carbonate (42.4 g; 0.4 moles) in methyl isobutyl
ketone (500 ml) was heated to the boiling point and a solution of
diphenylmethyl bromide (49.42 g; 0.2 moles) in methyl isobutyl ketone (75
ml) was slowly added in 1.5 hours. The resulting mixture was boiled under
reflux for another 12 hours, and then another solution of diphenylmethyl
bromide (24.71 g; 0.1 moles) in methyl isobutyl ketone (50 ml) was added
and the mixture boiled under reflux again for 12 hours. Another solution of
diphenylmethyl bromide in the same quantity was added and after refluxing
for 12 additional hours the reaction mixture was cooled, washed with water,
dried (Na2SO4) and the solvent removed in vacuum.
The residual oil was treated with the stoichiometric amount of fumaric acid in
ethanol and 4-diphenylmethoxy-1-[3-(4-tert-butylbenzoyl)propyl]piperidine
fumarate crystallized. After recrystallisation from ethanol the pure compound
was obtained (88 g; yield 75%), melting point 197-198°C.
brand name
Kestine (Rhone-Poulenc Rorer);Ebastel.
Therapeutic Function
Antihistaminic, Antiallergic, Calcium entry blocker
General Description
Ebastine is metabolised by cytochrome P450 3A (CYP3A4) to carebastine. It is used to treat allergic rhinitis and chronic idiopathic urticaria.
Biochem/physiol Actions
Ebastine is a non-sedating histamine H1 receptor antagonist, which inhibits allergen-induced bronchospasm in conscious guinea pigs. Unlike other compounds in this category, ebastine does not prolong the QT interval at up to five times the recommended therapeutic dose.
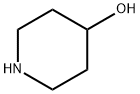

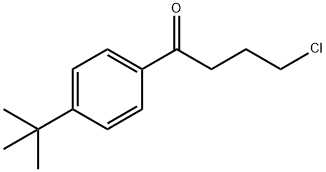

Ebastine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Shenzhen Excellent Biotech Co., Ltd. | 13480692018 | ramyan@ex-biotech.com | CHINA | 954 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569262 +86-15319487004 | 1015@dideu.com | China | 2127 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
View Lastest Price from Ebastine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Ebastine
90729-43-4
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min | 1000KG | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Ebastine
90729-43-4
|
US $47.00 / mg | 99.93% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-05 | Ebastine
90729-43-4
|
US $1.00-1.00 / KG | 1g | 99% | 50tons | Shaanxi Dideu Medichem Co. Ltd |





