Mycophenolic acid
- CAS No.
- 24280-93-1
- Chemical Name:
- Mycophenolic acid
- Synonyms
- Mycophenolic;melbex;nsc-129185;MycophenoL;Mycophenolate Mofetil EP Impurity F;(E)-6-(4-hydroxy-6-Methoxy-7-Methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-Methylhex-4-enoic acid;68618;lilly-68618;Mycophenolinsure;Micophenolic acid
- CBNumber:
- CB8311003
- Molecular Formula:
- C17H20O6
- Molecular Weight:
- 320.34
- MDL Number:
- MFCD00036814
- MOL File:
- 24280-93-1.mol
- MSDS File:
- SDS
| Melting point | 141°C |
|---|---|
| Boiling point | 419.24°C (rough estimate) |
| Density | 1.2300 (rough estimate) |
| vapor pressure | 0Pa at 22℃ |
| refractive index | 1.5200 (estimate) |
| Flash point | 2℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | methanol: 50 mg/mL, clear, colorless to faintly yellow |
| form | powder |
| pka | 4.5(at 25℃) |
| color | white to white with yellow cast |
| Water Solubility | 13mg/L(25 ºC) |
| Merck | 14,6327 |
| BRN | 1295848 |
| BCS Class | 2? |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| LogP | -1.83-2.28 at 25℃ and pH5-9 |
| Dissociation constant | 4.58-8.05 at 22.5-25℃ |
| CAS DataBase Reference | 24280-93-1(CAS DataBase Reference) |
| FDA UNII | HU9DX48N0T |
| NCI Drug Dictionary | mycophenolic acid |
| ATC code | L04AA06 |
| EPA Substance Registry System | 4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, (4E)- (24280-93-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H341-H360D-H372-H410 | |||||||||
| Precautionary statements | P201-P202-P260-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | Xn,N,T,F | |||||||||
| Risk Statements | 22-61-40-68-50/53-48/25-52/53-36-20/21/22-11 | |||||||||
| Safety Statements | 53-45-36/37-61-22-26-16 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MP8050000 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29419090 | |||||||||
| Toxicity | LD50 in mice (mg/kg): >1250 orally; 972.9±77 i.p. (Williams) | |||||||||
| NFPA 704 |
|
Mycophenolic acid price More Price(67)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M-106 | Mycophenolic acid solution 1.0?mg/mL in acetonitrile, ampule of 1?mL, certified reference material, Cerilliant? | 24280-93-1 | 1mL | $263 | 2024-03-01 | Buy |
| Sigma-Aldrich | 475913 | Mycophenolic Acid An immunosuppressive agent that inhibits | 24280-93-1 | 100mg | $125 | 2024-03-01 | Buy |
| Sigma-Aldrich | 475913 | Mycophenolic Acid An immunosuppressive agent that inhibits | 24280-93-1 | 500mg | $448 | 2024-03-01 | Buy |
| TCI Chemical | M2216 | Mycophenolic Acid >98.0%(HPLC)(T) | 24280-93-1 | 1g | $98 | 2024-03-01 | Buy |
| Alfa Aesar | J61905 | Mycophenolic acid, 98% | 24280-93-1 | 1g | $41.65 | 2024-03-01 | Buy |
Mycophenolic acid Chemical Properties,Uses,Production
Description
Mycophenolic acid(24280-93-1), less accurately called mycophenolate, is a compound derived from Penicillium stoloniferum and related species. It can reversibly inhibit inosine monophosphate dehydrogenase (IMPDH), the enzyme that controls the rate of synthesis of guanine monophosphate in the de novo pathway of purine synthesis used in the proliferation of B and T lymphocytes. Mycophenolic acid also has antibacterial, anticancer, antifungal, and antiviral activities. These powerful properties have been exploited in studies on organ transplant survival, anti-proliferative, rheumatoid arthritis, and psoriasis. It was initially marketed as the prodrug mycophenolate mofetil (MMF) to improve oral bioavailability. More recently, the salt mycophenolate sodium has also been introduced. Mycophenolate mofetil is marketed under the trade name CellCept and mycophenolate sodium as Myfortic.
Discovered by an Italian medical scientist Bartolomeo Gosio in 1893, mycophenolic acid was the first antibiotic to be synthesised in pure and crystalline form. But its medical application was forgotten until two American scientists C.L. Alsberg and O.M. Black resynthesised it in 1912, and gave its chemical name. It was eventually found to be a broad-spectrum acting drug having antiviral, antifungal, antibacterial, anticancer, and antipsoriasis properties. The clinically usable drug Cellcept was developed by South African geneticist Anthony Allison and his wife Elsie M. Eugui. It was first approved by the US Food and Drug Administration on 3 May 1995 for use in kidney transplantation.
Biosynthesis
The antibiotic, mycophenolic acid, biosynthesised by Penicillium brevicompactum, is unusual in that it is derived from a Cs polyketide chain and three dimethylallyl pyrophosphate molecules as shown in Figure 1.
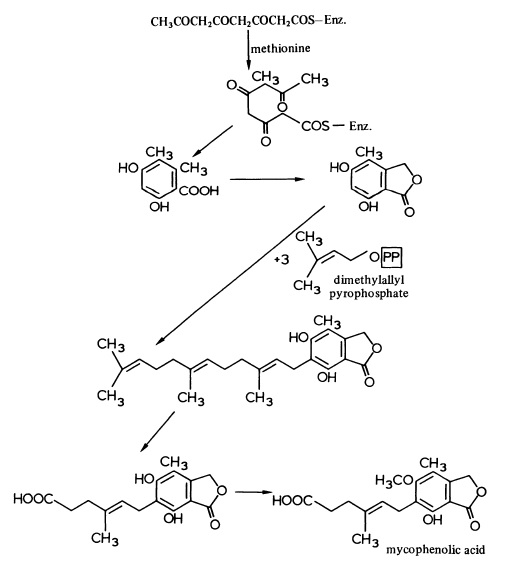
Mycophenolic acid has useful antitumour properties and efforts have been made to prepare derivatives with enhanced or modified activity. It is of inte rest ,therefore, that cultures of P. brevicompactum can convert halogenated phthalides (4.58; R = Cl or Br) into the corresponding mycophenolic acid derivatives.
References
1. https://pubchem.ncbi.nlm.nih.gov/compound/mycophenolic_acid#section=Top
2. https://www.scbt.com/scbt/product/mycophenolic-acid-24280-93-1
3. http://www.enzolifesciences.com/BML-A249/mycophenolic-acid/
4. http://www.emedicinehealth.com/drug-mycophenolic_acid/article_em.htm
5. https://pubchem.ncbi.nlm.nih.gov/compound/mycophenolic_acid#section=Top
6. http://www.selleckchem.com/products/Mycophenolic-acid(Mycophenolate).html
7. https://www.drugbank.ca/drugs/DB01024
Description
Mycophenolic acid is an immunosuppresive microbial metabolite that has been found in P. brevicompactum. It is also an active metabolite of mycophenolate mofetil that is formed via carboxylesterase 1 (CES1) and CES2. Mycophenolic acid is an inhibitor of IMP dehydrogenase (IMPDH) type I and type II (IC50s = 32 and 11 nM, respectively, in cell-free assays) and inhibits DNA synthesis in L strain mouse fibroblasts when used at concentrations ranging from 0.1 to 10 μg/ml. It is active against several strains of C. albicans, C. parakrusei, C. tropicalis, and C. neoformans (MICs = 3.9-31.25 μg/ml), as well as various strains of S. aureus (MICs = 31.25-125 μg/ml). Mycophenolic acid (150 mg/kg) reduces splenomegaly in a mouse model of Friend virus-induced leukemia. It decreases the number of hemolytic plaque forming cells isolated from the spleen of mice immunized with sheep red blood cells (RBCs) when administered at doses ranging from 60 to 240 mg/kg. Formulations containing mycophenolic acid have been used as immunosuppressive agents in the prevention of organ transplant rejection.
Chemical Properties
White to Off-White Powder
Uses
Mycophenolic acid is a common Penicillium metabolite first reported in the 1930s as a possible mycotoxin. Re-investigation showed mycophenolic acid to display broad antitumour, antiviral, antifungal and antiprotozoan activities. Its potent immunosuppressant activity led to its commercial development to prevent kidney transplant rejection. Mycophenolic acid acts by inhibiting inosine monophosphate dehydrogenase, controlling the rate of de novo purine synthesis in proliferating B and T lymphocytes.
Uses
antineoplastic, progestin
Uses
immune suppressant, antineoplastic, antiviral
Uses
An antibiotic produced by Penicillium brevi-compactum, P. Stoloniferum and related spp. A selective inhibitor of lymphocyte proliferation by blocking inosine monophosphate dehydrogenase, an enzyme involved in the de novo synthesis of purine nucleotides.
Definition
ChEBI: A member of the class of 2-benzofurans that is 2-benzofuran-1(3H)-one which is substituted at positions 4, 5, 6, and 7 by methyl, methoxy, (2E)-5-carboxy-3-methylpent-2-en-1-yl, and hydroxy groups, respectively. It is an antibiotic produced by Penicillium brevi-compactum, P. stoloniferum, P. echinulatum and related species. An immunosuppressant, it is widely used (partiularly as its sodium salt and as the 2-(morpholin-4-yl)ethyl ester prodrug, mycophenolate mo etil) to prevent tissue rejection following organ transplants and for the treatment of certain autoimmune diseases.
brand name
Myfortic (Novartis).
General Description
Assayed in therapeutic drug monitoring to ensure patients remain within the drug′s therapeutic range, mycophenolic acid is an immunosuppressant drug and the active metabolite of the prodrug mycophenolate mofetil. This analytical standard is suitable for LC-MS/MS applications including therapeutic drug monitoring and other clinical or diagnostic applications.
Biological Activity
Immunosuppressive agent with antiviral and antitumor effects in vitro and in vivo . Potently inhibits inosine monophosphate dehydrogenase, thus inhibiting de novo GTP synthesis leading to decreased RNA and DNA synthesis. Reversibly inhibits proliferation of T and B lymphocytes and antibody formation.
Mechanism of action
Mycophenolic acid (MPA) is a potent inhibitor of inosine monophosphate dehydrogenase (IMPDH) that prevents the ab initio biosynthesis of purine nucleotides. It predominantly affects lymphocytes, leading to inhibition of DNA synthesis in T cells and B cells, thereby suppressing cell-mediated immune responses and antibody formation.MPA also inhibits glycosylation and expression of adhesion molecules, as well as the recruitment of lymphocytes and monocytes to sites of inflammation.MPA depletes tetrahydrobiopterin and reduces nitric oxide production via inducible NO synthase without affecting the activity of constitutive NO synthase[1].
storage
Room temperature
Purification Methods
Purify the acid by dissolving it in the minimum volume of EtOAc, applying onto a silica gel column (0.05-0.2 mesh) and eluting with a mixture of EtOAc/CHCl3/AcOH (45:55:1) followed by recrystallisation from heptane/EtOAc, from aqueous EtOH or from hot H2O and drying in vacuo. It is a weak dibasic acid, moderately soluble in Et2O, CHCl3 and hot H2O but weakly soluble in *C6H6 and toluene. [Birch & Wright Aust J Chem 22 2635 1969, Canonica et al. J Chem Soc, Perkin Trans 1 2639 1972, Birkinshaw et al. Biochem J 50 630 1952, Beilstein 18 II 393, 18 III/IV 6513.]
References
[1] ALLISON A. Mechanisms of action of mycophenolate mofetil[J]. Lupus, 2005, 14 1: 2-8. DOI:10.1177/096120330501400102.
References
References/Citations 1) Eugui et al. (1991), Lymphocyte-selective cyostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion; Scand. J. Immunol., 33 161 2) Jonsson et al. (2002), Mycophenolic acid inhibits inosine 5′-monophosphate dehydrogenase and suppresses production of pro-inflammatory cytokines, nitric oxide and LDH in macrophages; Cell. Immunol., 216 93 3) Allison et al. (1993), Mechanisms of action of mycophenolic acid; Ann. NY Acad. Sci., 696 63 4) Quemeneur et al. (2002), Mycophenolic acid inhibits IL-2-dependent T cell proliferation, but not IL-2-dependent survival and sensitization to apoptosis; J. Immunol., 169 2747
Mycophenolic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Biopole Pharmatech Co., Ltd. | +8615151475053 | biopole@163.com | China | 37 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12815 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5871 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29861 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | sales@sdzschem.com | China | 2930 | 58 |
View Lastest Price from Mycophenolic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-11 | Mycophenolic acid
24280-93-1
|
US $0.00-0.00 / Kg/Drum | 1KG | 97%-103% | 500KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-03-31 | Mycophenolic acid
24280-93-1
|
US $1.00 / kg | 1kg | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-03-21 | Mycophenolic acid
24280-93-1
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd |
-

- Mycophenolic acid
24280-93-1
- US $0.00-0.00 / Kg/Drum
- 97%-103%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Mycophenolic acid
24280-93-1
- US $1.00 / kg
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Mycophenolic acid
24280-93-1
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd
24280-93-1(Mycophenolic acid )Related Search:
1of4





