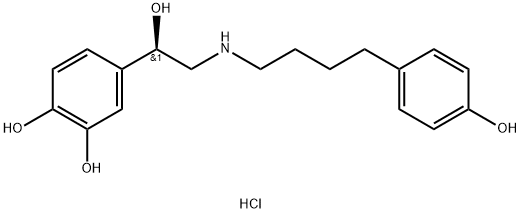
Arbutamine Hydrochloride
- CAS No.
- 125251-66-3
- Chemical Name:
- Arbutamine Hydrochloride
- Synonyms
- CBNumber:
- CB83159851
- Molecular Formula:
- C18H24ClNO4
- Molecular Weight:
- 353.84
- MDL Number:
- MFCD00902164
- MOL File:
- 125251-66-3.mol
| Melting point | 55-58° |
|---|---|
| alpha | D23 -18.5° (in ethanol) |
| FDA UNII | K0NF2CPJ7F |
Arbutamine Hydrochloride Chemical Properties,Uses,Production
Originator
Genesa,Genesa,UK
Manufacturing Process
(R)-Arbutamin was produced as follows: 89.3 mg of (-)-1-di(tbutyldimethylsiloxy)
phenyl)-2-aminoethanol, 50.0 mg of 4-(4-
methoxymethoxyphenyl)butanoic acid, diethylphosphorylcyanide, and
triethylamine were dissolved in N,N-dimethylformamide at 0°C, reacted at
room temperature, so as to obtain 108.6 mg (in a yield of 82%) of amide
compound. The amide compound obtained was reduced lithium aluminium
hydride in an ether solvent at reflux temperature, so as to quantitative obtain
amine. And 55.6 mg of (R)-arbutamin which is intended compound was
obtained by deprotecting the hydroxyl-protecting group of amine in a
methanol-THF solvent at room temperature using hydrochloric acid.
[α]D
25 = -17 (c 1.15, EtOH).
(-)-1-(Dibutyldimethylsiloxy)phenyl)-2-aminoethanol was obtained by a
hydrogenization of (R)-1-(3,4-di(t-butyldimethylsiloxyphenyl)-2-nitroethanol on 10% Pd/C.
The last compound was prepared as follows: the hydroxyl groups of 3,4-
dihydroxy-benzaldehyde was protected using t-butyldimethylsilylchloride (1).
100 mg (0.26 mmol) of 3,4-di(t-butyl-methylsiloxy)benzaldehyde was
dissolved in 1 ml tetrahydrofurane under atmosphere of argon at -40°C, and
0.30 ml of metal complex from (S)-6,6'-bis(triethylsilylethynyl)-1,1-dihydroxy-
2,2'-binaphtalene (2) mixed with a solution of n-butyllitium in hexane.After
stirring for 30 minutes, 79.4 mg (1.3 mmol) of nitromethane was added
dropwise to the mixture. After 67 hour reaction time, 2 ml of 1 N aqueous
solution of hydrochloric acid added to stop the reaction. Product was extracted
with 50 ml ethyl acetate, dehydrated with anhydrous sodium sulfate and
concentrated within evaporator followed by silica gel chromatography (nhexane/
acetone = 10/1), after which (R)-1-(3,4-di(t-butyldimethylsiloxy)
phenyl)-2-nitroethanol with an optical purity of 92% e.e. was obtained in a
yield of 93%.
Therapeutic Function
Cardiotonic
Arbutamine Hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
Arbutamine Hydrochloride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38631 | 58 | |
| TargetMol Chemicals Inc. | +8613564774135 | zijue.cai@tsbiochem.com | United States | 19884 | 58 |
| Pharmacodia (Beijing) Co.,Ltd | +86-400-851-9921 | sales@pharmacodia.com | China | 2317 | 55 |
| TargetMol Chemicals Inc. | 15002134094 | marketing@targetmol.cn | China | 19703 | 58 |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| TargetMol Chemicals Inc. | 58 |
| Pharmacodia (Beijing) Co.,Ltd | 55 |
| TargetMol Chemicals Inc. | 58 |
View Lastest Price from Arbutamine Hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-28 | Arbutamine hydrochloride
125251-66-3
|
US $1520.00-1980.00 / mg | 10g | TargetMol Chemicals Inc. |
-

- Arbutamine hydrochloride
125251-66-3
- US $1520.00-1980.00 / mg
- TargetMol Chemicals Inc.