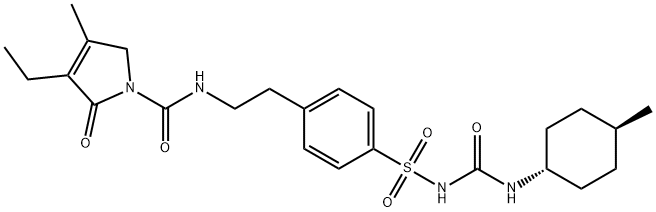
Glimepiride
- CAS No.
- 93479-97-1
- Chemical Name:
- Glimepiride
- Synonyms
- AMARYL;Glimpiride;GliMipride;N-(4-ethyl-3-methyl-5-oxo-2H-pyrrol-1-yl)-3-[4-[[[(4-methylcyclohexyl)amino]-oxomethyl]sulfamoyl]phenyl]propanamide;amary;hoe490;Grimepride;glimepirid;GliMperide;Glenn urea
- CBNumber:
- CB8318552
- Molecular Formula:
- C24H34N4O5S
- Molecular Weight:
- 490.62
- MDL Number:
- MFCD00878417
- MOL File:
- 93479-97-1.mol
| Melting point | 212.2-214.5 °C |
|---|---|
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: >10 mg/mL |
| pka | 5.10±0.10(Predicted) |
| form | solid |
| color | white |
| Merck | 14,4440 |
| BCS Class | 2 |
| CAS DataBase Reference | 93479-97-1(CAS DataBase Reference) |
| FDA UNII | 6KY687524K |
| NCI Drug Dictionary | Amaryl |
| ATC code | A10BB12 |
| EPA Substance Registry System | 1H-Pyrrole-1-carboxamide, 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans- 4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo- (93479-97-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361 | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 21-36/38-46-62-63 | |||||||||
| Safety Statements | 25-26-36/37-53 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UX9363950 | |||||||||
| HS Code | 2935904000 | |||||||||
| NFPA 704 |
|
Glimepiride price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | G2295 | Glimepiride ≥98% (HPLC), solid | 93479-97-1 | 50mg | $196 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP1144 | Glimepiride British Pharmacopoeia (BP) Reference Standard | 93479-97-1 | 100MG | $255 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP1141 | Glimepiride for system suitability British Pharmacopoeia (BP) Reference Standard | 93479-97-1 | 25MG | $251 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1292303 | Glimepiride United States Pharmacopeia (USP) Reference Standard | 93479-97-1 | 200mg | $678 | 2024-03-01 | Buy |
| TCI Chemical | G0395 | Glimepiride >98.0%(HPLC)(T) | 93479-97-1 | 1g | $99 | 2024-03-01 | Buy |
Glimepiride Chemical Properties,Uses,Production
Description
Glimepiride (original trade name Amaryl) is an orally available medium-to-long-acting sulfonylurea antidiabetic drug. It is sometimes classified as either the first third-generation sulfonylurea, or as second-generation. Like all sulfonylureas, glimepiride acts as an insulin secretagogue. It lowers blood sugar by stimulating the release of insulin from functioning pancreatic beta cells and by increasing sensitivity of peripheral tissues to insulin. Glimepiride likely binds to ATP-sensitive potassium channel receptors on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Membrane depolarization stimulates calcium ion influx through voltage-sensitive calcium channels. This increase in intracellular calcium ion concentration induces the secretion of insulin. Glimepiride is mainly used to treat patients with type 2 diabetes and can also decrease the chances that someone will develop complications of type 2 diabetes, such as kidney damage, blindness, nerve problems, loss of limbs, sexual function problems and heart attack or stroke. The drug was approved by the FDA in 1995 and is manufactured by Sanofi-Aventis. It can be used along with proper diet and exercise program and may also be used alone or with other antidiabetic medicines if need. The drug is available only with your doctor's prescription.
References
1. https://en.wikipedia.org/wiki/Glimepiride
2. http://www.webmd.com/drugs/2/drug-12271/glimepiride-oral/details
3. https://www.drugs.com/cdi/glimepiride.html
4. http://www.medicinenet.com/glimepiride/article.htm
5. http://www.everydayhealth.com/drugs/glimepiride
6. http://www.emedicinehealth.com/drug-glimepiride/article_em.htm
7. https://www.ghc.org/kbase/topic.jhtml?docId=d03864a1
8. http://www.emedicinehealth.com/drug-glimepiride/article_em.htm
9. http://drugs.healthgrove.com/l/3454/Glimepiride
Description
Glimepiride, the first of a new generation of sulfonylurea drugs, was introduced in Sweden in 1995 as a first-line therapy to lower blood glucose in patients with type II diabetes. Sulfonylureas exert their hypoglycemic function primarily by direct stimulation of insulin secretion in glucose-insensitive pancreatic β-cells and GLUT translocation in insulin-resistant fat and muscle cells. Once-daily, orally administered glimepiride in diabetes patients showed a more rapid and longer lasting glucose-lowering effect than the commonly used agent glibenclamide. Glimepiride can be used either as a monotherapy or in combination with insulin.
Chemical Properties
White Cyrstalline Solid
Originator
Hoechst Marion Roussel (Germany)
Uses
A sulfonylurea hypoglycemic agent. Used as an antidiabetic
Uses
Glimepiride induces the PI3 kinase (PI3K) and Akt pathway, along with insulin receptor substrate-1/2 and endothelial nitric oxide synthase. Glimepiride also increases osteoblast proliferation and differentiation, which is thought to be related to its ability to activate the PI3K and Akt pathway. Furthermore, Glimepiride enhances intrinsic peroxisome proliferator-activated receptor γ activity. Glimepiride also increases protein expression of glucose transports 1 and 4, and is a potent KIR channel blocker. Potent Kir6 (KATP) channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac Kir6 channels with an IC50 of 6.8 nM.
Uses
For concomitant use with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus.
Uses
anticonvulsant
Definition
ChEBI: Glimepiride is a sulfonamide, a N-acylurea and a N-sulfonylurea. It has a role as a hypoglycemic agent and an insulin secretagogue.
Manufacturing Process
By heating of a mixture of 3-ethyl-4-methyl-2-pyrrolone and 2- phenylethylisocyanate at 150°C is obtained 3-ethyl-4-methyl-2-oxo-3- pyrroline-1-(N-2-phenylethyl)-carboxamide, melting point 106°-108°C. Then the carboxamide are introduced in portions at 30°C into chlorosulfonic acid, and agitated for 1 hour at 40°C. The sulfochloride (melting point 172-175°C), introduced into concentrated ammonia, and heated for 30 min on a steam bath. The mixture of sulfonamide obtained (melting point 180°-182°C), of acetone and K2CO3 are refluxed with agitation for 6 hours. Subsequently the cyclohexyl isocyanate are added dropwise, and agitation is continued for 6 hours at boiling temperature. After standing overnight, the product is filtered, the crystals obtained are treated with dilute hydrochloric acid, and again filtered. It is prepared N-(4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1- carboxamido)ethyl]benzenesulfonyl)-N'-cyclohexyl urea; melting point 185°- 187°C (from acetone) (Glimepiride).
brand name
Amaryl (Sanofi Aventis).
Therapeutic Function
Oral hypoglycemic
General Description
Glimepiride, 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)urea (Amaryl), is very similarto glipizide with the exception of their heterocyclic rings.Instead of the pyrazine ring found in glipizide, glimepiridecontains a pyrrolidine system. It is metabolized primarilythrough oxidation of the alkyl side chain of the pyrrolidine,with a minor metabolic route involving acetylation of theamine.
General Description
Glimepiride is 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans-4-methylcyclohexyl)amino]-carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxamide; thiscompound can also be named as the urea—see precedingdiscussion (Amaryl, generic). Combinations are availablewith rosiglitazone in the United States (Avandaryl tablets;mg glimepiride/mg rosiglitazone as maleate salt: 1/4,2/4, 4/4, 2/8, 4/8); and with pioglitazone (Duetact tablets;mg glimepiride/ mg pioglitazone as hydrochloride salt:2/30, 4/30).
Biological Activity
Potent K ATP channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac K ATP channels with an IC 50 of 6.8 nM.
Biochem/physiol Actions
Glimepiride is a potent blocker of cardiac KATP channels activated by pinacidil with an IC50 of 6.8 nM.
Clinical Use
Non-insulin dependent diabetes mellitus
Veterinary Drugs and Treatments
Glimepiride may potentially be a useful adjunct in the treatment of non-insulin dependent diabetes mellitus (NIDDM) in cats. Its duration of action in humans allows it to be dosed once daily, which could be of benefit in cats. It may also have fewer side effects than glipizide in cats.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs.
Antibacterials: effects enhanced by chloramphenicol,
sulphonamides, tetracyclines and trimethoprim;
effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by
coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole
and miconazole and possibly voriconazole.
Lipid-regulating drugs: possibly additive
hypoglycaemic effect with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas.
Metabolism
The drug is extensively metabolised in the liver to two
main metabolites. The cytochrome P450 isoenzyme
CYP2C9 is involved in the formation of a hydroxy
derivative, which is further metabolised to a carboxy
derivative by cytosolic enzymes.
About 60% of a dose is eliminated in the urine and 40%
in the faeces.
Glimepiride Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12815 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5871 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18137 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3007 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 | linda@tnjone.com | China | 1143 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21630 | 55 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 | bruce@xrdchem.cn | CHINA | 566 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
View Lastest Price from Glimepiride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-11 | Glimepiride
93479-97-1
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%-102%;USP | 100KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-02 | Glimepiride
93479-97-1
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2025-03-31 | Glimepiride
93479-97-1
|
US $10.00 / KG | 1KG | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd |
-

- Glimepiride
93479-97-1
- US $0.00-0.00 / Kg/Drum
- 98%-102%;USP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Glimepiride
93479-97-1
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Glimepiride
93479-97-1
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
93479-97-1(Glimepiride)Related Search:
1of4