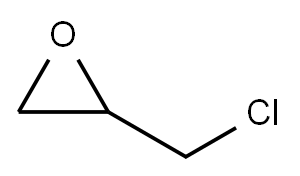
Epichlorohydrin
- CAS No.
- 106-89-8
- Chemical Name:
- Epichlorohydrin
- Synonyms
- ECH;2-(Chloromethyl)oxirane;EPICHLOROHYDRINE;EPICHLORHYDRIN;1-CHLORO-2,3-EPOXYPROPANE;Epichlorhydrine;ALPHA-EPICHLOROHYDRIN;New product 99.9% purity CAS 106-89-8 Epichlorohydrin CAS NO.106-89-8 Manufacturers wholesale;Epicloridrina;J006
- CBNumber:
- CB8381781
- Molecular Formula:
- C3H5ClO
- Molecular Weight:
- 92.52
- MDL Number:
- MFCD00005132
- MOL File:
- 106-89-8.mol
- MSDS File:
- SDS
| Melting point | -57 °C |
|---|---|
| alpha | -1~+1°(D/20℃)(c=1,CH3OH) |
| Boiling point | 115-117 °C(lit.) |
| Density | 1.183 g/mL at 25 °C(lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 13.8 mm Hg ( 21.1 °C) |
| refractive index |
n |
| Flash point | 93 °F |
| storage temp. | Store below +30°C. |
| solubility | 65.9g/l |
| form | Liquid |
| color | APHA: ≤20 |
| Specific Gravity | 1.183 (20/4℃) |
| Odor | Pungent, garlic; sweet, pungent; like chloroform. |
| explosive limit | 3.8-21%(V) |
| Water Solubility | 6 g/100 mL (10 ºC) |
| FreezingPoint | -57.2℃ |
| Merck | 14,3611 |
| BRN | 79785 |
| Henry's Law Constant | 3.42(x 10-5 atm?m3/mol) at 25 °C (static headspace-GC, Welke et al., 1998) |
| Exposure limits | TLV-TWA(skin) 8 mg/m3 (2 ppm) (ACGIH); STEL (15 min) 19 mg/m3 (5 ppm) (NIOSH). |
| Dielectric constant | 22.9(20℃) |
| Stability | Unstable. Flammable - note wide explosion limits and low flash point. |
| EPA Primary Drinking Water Standard | MCL:TT4,MCLG:zero |
| LogP | 0.45 at 20℃ |
| Indirect Additives used in Food Contact Substances | EPICHLOROHYDRIN |
| FDA 21 CFR | 176.170 |
| CAS DataBase Reference | 106-89-8(CAS DataBase Reference) |
| EWG's Food Scores | 10 |
| FDA UNII | 08OOR508C0 |
| Proposition 65 List | Epichlorohydrin |
| IARC | 2A (Vol. 11, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Oxirane, (chloromethyl)-(106-89-8) |
| EPA Substance Registry System | Epichlorohydrin (106-89-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H301+H311+H331-H314-H317-H350 | |||||||||
| Precautionary statements | P201-P210-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 45-10-23/24/25-34-43 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | UN 2023 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TX4900000 | |||||||||
| Autoignition Temperature | 779 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2910 30 00 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 106-89-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 0.09 g/kg (Smyth, Carpenter) | |||||||||
| IDLA | 75 ppm | |||||||||
| NFPA 704 |
|
Epichlorohydrin price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.03296 | Epichlorohydrine forsynthesis | 106-89-8 | 100mL | $56.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03296 | Epichlorohydrine forsynthesis | 106-89-8 | 30kg | $901 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.03296 | Epichlorohydrine forsynthesis | 106-89-8 | 60kg | $1590 | 2024-03-01 | Buy |
| Sigma-Aldrich | 45340 | (±)-Epichlorohydrin purum,≥99%(GC) | 106-89-8 | 500mL | $43.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 45340 | (±)-Epichlorohydrin purum,≥99%(GC) | 106-89-8 | 1L | $65.5 | 2024-03-01 | Buy |
Epichlorohydrin Chemical Properties,Uses,Production
Description
Epichlorohydrin is a kind of organochlorine compound as well as epoxide. It can be used as an industrial solvent. It is a highly reactive compound, and can be used for the production of glycerol, plastics, epoxy glues and resins, and elastomers. It can also be used for the production of glycidyl nitrate and alkali chloride, used as the solvent of cellulose, resins, and paint as well as being used as an insect fumigant. In biochemistry, it can be used as a crosslinking agent for the production of Sephdex size-exclusion chromatography resins. However, it is a potential carcinogen, and can cause various kinds of side effects on respiratory tract and kidneys. It can be manufactured through the reaction between allyl chloride with hypochlorous acid as well as alcohols.
Chemical Properties
Epichlorohydrin (molecular weight = 92.53 g/mol) is a colorless liquid with a sweet or garlic-like pungent odor. It is soluble in water (6.6 × 104 mg/l at 25 °C) and miscible with most organic solvents.
Uses
Epichlorohydrin is mainly used in the production of epoxy resins. It was also used as a solvent for paints, varnishes, lacquers, cellulose esters and ethers, and gums. Epichlorohydrin was historically used as an insecticide fumigant.
Environmental Fate
Epichlorohydrin may be found in the environment as a result of release in waste streams of resin, paint and lacquer, cellulose ester/ether, and gum production. The high vapor pressure of epichlorohydrin maintains it as a gas in the atmosphere, where it is degraded by photochemically produced hydroxyl radicals (half-life=36 days). Epichlorohydrin is highly mobile in soil, evaporates quickly from dry soil, and is extensively hydrolyzed in moist soil and water. It is not expected to be adsorbed to sediments and suspended particles and is not expected to bioaccumulate in aquatic organisms(NLM, 2013).
References
https://en.wikipedia.org/wiki/Epichlorohydrin
https://pubchem.ncbi.nlm.nih.gov/compound/epichlorohydrin#section=Top
Description
Epoxy resins of the bisphenol A type are synthesized from epichlorhydrin and bisphenol A. This leads to bisphenol-A diglycidyl ether, which is the monomer of bisphenol-A-based epoxy resins. Sensitization to epichlorhydrin occurs mainly in workers in the epoxy-resin industry. Sensitization in individuals not working at epoxy resin plants is rare. It has however been described to occur after contact with a soil fumigant, due to solvent cement and in a worker in a pharmaceutical plant, in a division for drug synthesis. Epichlorhydrin was used for the production of both drugs propranolol and oxprenolol.
Chemical Properties
Epichlorohydrin is a colorless liquid with a slightly irritating, chloroform-like odor.
Physical properties
Clear, colorless, mobile liquid with a strong, irritating, chloroform-like odor. Odor threshold concentration is 0.93 ppm (quoted, Amoore and Hautala, 1983).
Uses
Commercially the most important use is production of glycerine. Large volumes are consumed in nonglycerine areas, which largely consist of the various epoxy resins. It has use as a solvent and in the production of epichlorohydrin rubber.
Uses
Epichlorohydrin is used to make glycerol,epoxy resins, adhesive, and castings; asderivatives for producing dyes, pharmaceu-ticals, surfactants, and plasticizers; and asa solvent for resins, gums, paints, andvarnishes.
Uses
Solvent for natural and synthetic resins, gums, cellulose esters and ethers, paints, varnishes, nail enamels and lacquers, cement for Celluloid. As stabilizer.
Definition
ChEBI: An epoxide that is 1,2-epoxypropene in which one of the methyl hydrogens is substituted by chlorine.
Production Methods
Epichlorohydrin can be prepared from 1,3-dichloropropanol-2, 2,3- dichloropropanol-1, or allyl chloride. Commercially it is prepared as an intermediate in glycerol synthesis via alkaline hydrolysis of glycerol dichlorohydrin. Both come from allyl chloride. Epichlorohydrin reacts with monohydric alcohols to give ethers by opening the oxide ring. It will react with ethers, aldehydes, ketones, organic acids and amines to give a wide variety of useful syntheses.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 2, p. 256, 1943
The Journal of Organic Chemistry, 48, p. 3831, 1983 DOI: 10.1021/jo00169a052
General Description
A clear colorless liquid with an irritating chloroform-like odor. Density 9.8 lb / gal. Flash point 87°F. Polymerizable. If polymerization takes place inside a closed container, the container is subject to violent rupture. Irritates the skin and respiratory system. Toxic by ingestion. A confirmed carcinogen. Vapors heavier than air. Used to make plastics and as a solvent.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Epichlorohydrin may polymerize exothermically if heated or contaminated. Reacts explosively with aniline. Ignites on contact with potassium tert-butoxide. Reacts with trichloroethylene to give the explosive dichloroacetylene. Violent reaction with sulfuric acid or isopropylamine. Exothermic polymerization on contact with strong acids or bases, zinc, aluminum, aluminum chloride, iron, ferric chloride [Sax, 9th ed., 1996, p. 1469].
Hazard
Toxic by inhalation, ingestion, and skin absorption; strong irritant, a carcinogen. Flammable, moderate fire risk. TLV: 0.5 ppm; animal carcinogen.
Health Hazard
Epichlorohydrin is toxic, carcinogenic, and astrong irritant. Its vapors can produce irrita-tion in the eyes, skin, and respiratory tract.Exposure to high concentration resulted indeath in animals, injuring the central nervoussystem. The liquid can absorb through humanskin, causing painful irritation of subcuta-neous tissues (ACGIH 1986). The symptomsof toxicity from high dosage in test animalswere paralysis of muscles and slow devel-opment of respiratory distress. Long expo-sures at 120 ppm for several hours resultedin lung, kidney, and liver injury in rats(Gage 1959). Ingestion by an oral routecaused tremor, somnolence, and ataxia inmice (NIOSH 1986). The toxic symptomsand lethal doses varied widely with animalspecies. The toxic metabolite of epichlorhy-drin could be ?- chlorohydrin ; thelatter was produced in vitro by rat livermicrosomes (Gingell et al. 1987).
A 25 ppm concentration may be detectableby odor. Exposure at this level may causeburning of the eyes and nose in humans.Above 100 ppm even a short exposure maybe hazardous to humans, causing nausea,dyspnea, lung edema, and kidney injury.
Epichlorohydrin is mutagenic and hasshown carcinogenicity in test animals. Itcaused tumors in the lungs and nose andat gastrointestinal and endocrine sites. Expo-sure to this compound caused harmful repro-ductive effects on fertility and birth defectsin mice.
Health Hazard
Epichlorohydrin is caustic as both a liquid and gas. Irritation of the eyes and skin, and skin sensitization has been observed. Exposure to epichlorohydrin has caused inflammation of the lungs, asthmatic bronchitis, and liver and kidney damage. In acute poisonings, death may be caused by respiratory paralysis.
Fire Hazard
When heated to decomposition, Epichlorohydrin evolves highly toxic fumes of phosgene and carbon monoxide. Reactive and incompatible with strong oxidizers, strong acids, caustics, zinc, aluminum, chlorides of iron and aluminumand compounds with an active hydrogen atom, including water. Unstable, avoid heat, contaminants, strong acids and bases, certain curing agents such as ethylenediamine. Hazardous polymerization may occur.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water Mild reaction; not likely to be hazardous; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Can polymerize in presence of strong acids and bases, particularly when hot; Inhibitor of Polymerization: None used.
Contact allergens
Epoxy resin of the Bisphenol A type is synthesized from epichlorhydrin and bisphenol A. It leads to bisphenol A diglycidyl ether, which is the monomer ofbisphenol-A-based epoxy resins. Sensitization to epichlorhydrin occurs mainly in workers of the epoxy resin industry. Sensitization in individuals not working at epoxy resin plants is rare. It has, however, been described to occur following exposure to a soil fumigant, due to solvent cement, and in a worker in a pharmaceutical plant, in a division of drug synthesis. Epichlorhydrin was used for the production of drugs propranolol and oxprenolol.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion, skin contact, intravenous, and intraperitoneal routes. Moderately toxic by inhalation. An experimental teratogen. Other experimental reproductive effects. Human systemic effects by inhalation: respiratory, nose, and eyes. Human mutation data reported. A skin and eye irritant. A sensitizer. Flammable liquid when exposed to heat or flame. Explosive reaction with andine. Reaction with trichloroethylene forms the explosive dichloroacetylene. Ignition on contact with potassium tertbutoxide. Violent reaction with sulfuric acid or isopropylamine. Exothermic polymerization on contact with strong acids, caustic alkalies, aluminum, aluminum chloride, iron(II1) chloride, or zinc. When heated to decomposition it emits toxic fumes of Cl
Potential Exposure
Epichlorohydrin, an organochlorine, is used in the manufacture of many glycerol and glycidol derivatives and epoxy resins; as a stabilizer in chlorine-containing materials; as an intermediate in the preparation of cellulose esters and ethers, paints, varnishes, nail enamels, and lacquers; as a cement for celluloid. It is used as an intermediate in the manufacture of various drugs. Increased cancer risk.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray
Carcinogenicity
Epichlorohydrin is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Biological. Bridié et al. (1979) reported BOD and COD values of 0.03 and 1.16 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C for a period of 5 d. When a sewage seed was used in a
separate screening test, a BOD value of 0.16 g/g was obtained. The ThOD for epichlorohydrin is
1.21 g/g.
Chemical/Physical. Anticipated products from the reaction of epichlorohydrin with ozone or
OH radicals in the atmosphere are formaldehyde, glyoxylic acid, and ClCH2O(O)OHCHO (Cupitt,
1980). Haag and Yao (1992) reported a calculated OH radical rate constant in water of 2.9 x
108/M?sec.
storage
Epichlorohydrin is stored in a well-ventilated,cool place isolated from combustible andoxidizable materials, all acids and bases,and anhydrous metal halides. Protect fromphysical damage. It is shipped in metaldrums.
Shipping
UN2023 Epichlorhydrin, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.
Purification Methods
Distil epichlorohydrin under atmospheric pressure, heat it on a steam bath with one-quarter its weight of CaO, then decant and fractionally distil it. [Beilstein 17 V 20.]
Toxicity evaluation
Epichlorohydrin is an alkylating agent that is mutagenic. It may induce DNA interstrand cross-links, chromosomal aberrations, and breaks. It is also an irritant, sensitizer, and corrosive.
Incompatibilities
May form explosive mixture with air. Slowly decomposes on contact with water. Heat or strong acids; alkalies, metallic halides, or contaminants can cause explosive polymerization. Violent reaction with strong oxidizers, aliphatic amines; alkanolamines, amines (especially aniline), alkaline earths; chemically active metals (chlorides of aluminum, iron zinc); powdered metals (aluminum, zinc); alcohols, phenols, organic acids; causing fire and explosion hazard. Will pit steel in the presence of water. Thermal decomposition forms highly toxic phosgene gas. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
Epichlorohydrin Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 | sales002@sdzschem.com | China | 3049 | 58 |
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 | sales@yanshuochem.com | China | 101 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; | peter68@ptchemgroup.com | China | 35425 | 58 |
| Wuxi High Mountain Hi-tech Development Co.,Ltd. | +86-86-0510-85881806 +8613357920996 | wuxihighmountain@gmail.com | China | 30 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20284 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 990 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 | sales@qdrenas.com | China | 476 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
Related articles
- Synthesis and Uses of Epichlorohydrin
- Epichlorohydrin (ECH) is an important organic chemical raw material and petrochemical intermediate, mainly used to produce epo....
- Jun 1,2022
- The Application of Epichlorohydrin
- Epichlorohydrin is manufactured from allyl chloride and is widely used as a chemical intermediate. A major use is in the synth....
- Oct 19,2021
- General Properties of Epichlorohydrin
- Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. It is a colorless liquid with a pungent, garli....
- Nov 8,2019
View Lastest Price from Epichlorohydrin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-14 | Epichlorohydrin
106-89-8
|
US $80.00-2000.00 / kg | 1000kg | 0.995 | 50000MT per year | Wuxi High Mountain Hi-tech Development Co.,Ltd. | |
 |
2024-10-29 | Epichlorohydrin
106-89-8
|
US $0.00 / kg | 20kg | 99.0% | 20 tons | Qingdao RENAS Polymer Material Co., Ltd. | |
 |
2024-10-11 | Epichlorohydrin
106-89-8
|
US $10.00 / KG | 1KG | 99% | 50 ton | Hebei Chuanghai Biotechnology Co,.LTD |
-

- Epichlorohydrin
106-89-8
- US $80.00-2000.00 / kg
- 0.995
- Wuxi High Mountain Hi-tech Development Co.,Ltd.
-

- Epichlorohydrin
106-89-8
- US $0.00 / kg
- 99.0%
- Qingdao RENAS Polymer Material Co., Ltd.
-

- Epichlorohydrin
106-89-8
- US $10.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD