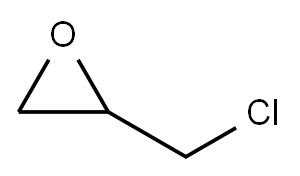
에피클로로하이드린
|
|
에피클로로하이드린 속성
- 녹는점
- -57 °C
- 알파
- -1~+1°(D/20℃)(c=1,CH3OH)
- 끓는 점
- 115-117 °C(lit.)
- 밀도
- 1.183 g/mL at 25 °C(lit.)
- 증기 밀도
- 3.2 (vs air)
- 증기압
- 13.8 mm Hg ( 21.1 °C)
- 굴절률
- n
20/D 1.438(lit.)
- 인화점
- 93 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 65.9g/L
- 물리적 상태
- 액체
- 색상
- APHA: ≤20
- Specific Gravity
- 1.183 (20/4℃)
- 냄새
- 매운맛, 마늘; 달콤하고 매운; 클로로포름처럼요.
- 폭발한계
- 3.8-21%(V)
- 수용성
- 6 g/100 mL (10 ºC)
- 어는점
- -57.2℃
- Merck
- 14,3611
- BRN
- 79785
- Henry's Law Constant
- 3.42(x 10-5 atm?m3/mol) at 25 °C (static headspace-GC, Welke et al., 1998)
- 노출 한도
- TLV-TWA(skin) 8 mg/m3 (2 ppm) (ACGIH); STEL (15 min) 19 mg/m3 (5 ppm) (NIOSH).
- Dielectric constant
- 22.9(20℃)
- 안정성
- 불안정한. 가연성 - 넓은 폭발 한계와 낮은 인화점에 유의하십시오.
- LogP
- 0.45 at 20℃
- CAS 데이터베이스
- 106-89-8(CAS DataBase Reference)
- IARC
- 2A (Vol. 11, Sup 7, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-10-23/24/25-34-43 | ||
| 안전지침서 | 53-45 | ||
| 유엔번호(UN No.) | UN 2023 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TX4900000 | ||
| 자연 발화 온도 | 779 °F | ||
| TSCA | Yes | ||
| HS 번호 | 2910 30 00 | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 106-89-8(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 0.09 g/kg (Smyth, Carpenter) | ||
| IDLA | 75 ppm | ||
| 기존화학 물질 | KE-05647 | ||
| 유해화학물질 필터링 | 97-1-192 | ||
| 중점관리물질 필터링 | 별표1-54 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 에피클로로히드린 및 이를 0.1% 이상 함유한 혼합물 |
에피클로로하이드린 C화학적 특성, 용도, 생산
용도
인조 수지 : 에폭시 수지, 열경화성 수지, 이온 교환 수지. 의학 : 글리세린, 마취약, 해열제, 진정제 고무 :에피클로로 고무 농예화학 : 살충제, 살균제 기 타 : 세척제, 솔벤트, 안정제, 계면활성제.안전성
325℃은 위험하므로 취급주의.- 열이나 불, 스파크에 노출시 폭발 위험.
- 수분을 흡수할 수 있으므로 건조하게 보관.
- 외부 충격으로부터 보호.
- 이물질을 피해서 보관.
개요
Epoxy resins of the bisphenol A type are synthesized from epichlorhydrin and bisphenol A. This leads to bisphenol-A diglycidyl ether, which is the monomer of bisphenol-A-based epoxy resins. Sensitization to epichlorhydrin occurs mainly in workers in the epoxy-resin industry. Sensitization in individuals not working at epoxy resin plants is rare. It has however been described to occur after contact with a soil fumigant, due to solvent cement and in a worker in a pharmaceutical plant, in a division for drug synthesis. Epichlorhydrin was used for the production of both drugs propranolol and oxprenolol.화학적 성질
Epichlorohydrin is a colorless liquid with a slightly irritating, chloroform-like odor.물리적 성질
Clear, colorless, mobile liquid with a strong, irritating, chloroform-like odor. Odor threshold concentration is 0.93 ppm (quoted, Amoore and Hautala, 1983).용도
Epichlorohydrin is used to make glycerol,epoxy resins, adhesive, and castings; asderivatives for producing dyes, pharmaceu-ticals, surfactants, and plasticizers; and asa solvent for resins, gums, paints, andvarnishes.생산 방법
Epichlorohydrin can be prepared from 1,3-dichloropropanol-2, 2,3- dichloropropanol-1, or allyl chloride. Commercially it is prepared as an intermediate in glycerol synthesis via alkaline hydrolysis of glycerol dichlorohydrin. Both come from allyl chloride. Epichlorohydrin reacts with monohydric alcohols to give ethers by opening the oxide ring. It will react with ethers, aldehydes, ketones, organic acids and amines to give a wide variety of useful syntheses.정의
ChEBI: An epoxide that is 1,2-epoxypropene in which one of the methyl hydrogens is substituted by chlorine.일반 설명
A clear colorless liquid with an irritating chloroform-like odor. Density 9.8 lb / gal. Flash point 87°F. Polymerizable. If polymerization takes place inside a closed container, the container is subject to violent rupture. Irritates the skin and respiratory system. Toxic by ingestion. A confirmed carcinogen. Vapors heavier than air. Used to make plastics and as a solvent.공기와 물의 반응
Highly flammable. Water soluble.반응 프로필
Epichlorohydrin may polymerize exothermically if heated or contaminated. Reacts explosively with aniline. Ignites on contact with potassium tert-butoxide. Reacts with trichloroethylene to give the explosive dichloroacetylene. Violent reaction with sulfuric acid or isopropylamine. Exothermic polymerization on contact with strong acids or bases, zinc, aluminum, aluminum chloride, iron, ferric chloride [Sax, 9th ed., 1996, p. 1469].위험도
Toxic by inhalation, ingestion, and skin absorption; strong irritant, a carcinogen. Flammable, moderate fire risk. TLV: 0.5 ppm; animal carcinogen.건강위험
Epichlorohydrin is caustic as both a liquid and gas. Irritation of the eyes and skin, and skin sensitization has been observed. Exposure to epichlorohydrin has caused inflammation of the lungs, asthmatic bronchitis, and liver and kidney damage. In acute poisonings, death may be caused by respiratory paralysis.화재위험
When heated to decomposition, Epichlorohydrin evolves highly toxic fumes of phosgene and carbon monoxide. Reactive and incompatible with strong oxidizers, strong acids, caustics, zinc, aluminum, chlorides of iron and aluminumand compounds with an active hydrogen atom, including water. Unstable, avoid heat, contaminants, strong acids and bases, certain curing agents such as ethylenediamine. Hazardous polymerization may occur.화학 반응
Reactivity with Water Mild reaction; not likely to be hazardous; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Can polymerize in presence of strong acids and bases, particularly when hot; Inhibitor of Polymerization: None used.색상 색인 번호
Epoxy resin of the Bisphenol A type is synthesized from epichlorhydrin and bisphenol A. It leads to bisphenol A diglycidyl ether, which is the monomer ofbisphenol-A-based epoxy resins. Sensitization to epichlorhydrin occurs mainly in workers of the epoxy resin industry. Sensitization in individuals not working at epoxy resin plants is rare. It has, however, been described to occur following exposure to a soil fumigant, due to solvent cement, and in a worker in a pharmaceutical plant, in a division of drug synthesis. Epichlorhydrin was used for the production of drugs propranolol and oxprenolol.Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion, skin contact, intravenous, and intraperitoneal routes. Moderately toxic by inhalation. An experimental teratogen. Other experimental reproductive effects. Human systemic effects by inhalation: respiratory, nose, and eyes. Human mutation data reported. A skin and eye irritant. A sensitizer. Flammable liquid when exposed to heat or flame. Explosive reaction with andine. Reaction with trichloroethylene forms the explosive dichloroacetylene. Ignition on contact with potassium tertbutoxide. Violent reaction with sulfuric acid or isopropylamine. Exothermic polymerization on contact with strong acids, caustic alkalies, aluminum, aluminum chloride, iron(II1) chloride, or zinc. When heated to decomposition it emits toxic fumes of Cl잠재적 노출
Epichlorohydrin, an organochlorine, is used in the manufacture of many glycerol and glycidol derivatives and epoxy resins; as a stabilizer in chlorine-containing materials; as an intermediate in the preparation of cellulose esters and ethers, paints, varnishes, nail enamels, and lacquers; as a cement for celluloid. It is used as an intermediate in the manufacture of various drugs. Increased cancer risk.Carcinogenicity
Epichlorohydrin is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.환경귀착
Biological. Bridié et al. (1979) reported BOD and COD values of 0.03 and 1.16 g/g using filtered effluent from a biological sanitary waste treatment plant. These values were determined using a standard dilution method at 20 °C for a period of 5 d. When a sewage seed was used in a separate screening test, a BOD value of 0.16 g/g was obtained. The ThOD for epichlorohydrin is 1.21 g/g.Chemical/Physical. Anticipated products from the reaction of epichlorohydrin with ozone or OH radicals in the atmosphere are formaldehyde, glyoxylic acid, and ClCH2O(O)OHCHO (Cupitt, 1980). Haag and Yao (1992) reported a calculated OH radical rate constant in water of 2.9 x 108/M?sec.
저장
Epichlorohydrin is stored in a well-ventilated,cool place isolated from combustible andoxidizable materials, all acids and bases,and anhydrous metal halides. Protect fromphysical damage. It is shipped in metaldrums.운송 방법
UN2023 Epichlorhydrin, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid.Purification Methods
Distil epichlorohydrin under atmospheric pressure, heat it on a steam bath with one-quarter its weight of CaO, then decant and fractionally distil it. [Beilstein 17 V 20.]비 호환성
May form explosive mixture with air. Slowly decomposes on contact with water. Heat or strong acids; alkalies, metallic halides, or contaminants can cause explosive polymerization. Violent reaction with strong oxidizers, aliphatic amines; alkanolamines, amines (especially aniline), alkaline earths; chemically active metals (chlorides of aluminum, iron zinc); powdered metals (aluminum, zinc); alcohols, phenols, organic acids; causing fire and explosion hazard. Will pit steel in the presence of water. Thermal decomposition forms highly toxic phosgene gas. May accumulate static electrical charges, and may cause ignition of its vapors.폐기물 처리
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal에피클로로하이드린 준비 용품 및 원자재
원자재
디클로로(2,3-)-1-프로판올
탄산칼슘
2-Amino-3-chlorobenzoic acid
프로필렌
염소(기체)
산소, 냉각된 액체
D-D Extender
아세트산(빙초산)
수산화나트륨
하이포클로로스애씨드
염화알릴
탄산나트륨(경회)
준비 용품
4-(옥시라닐메톡시)-1H-인돌
CARAZOLOL HCL
Finishing agent FS
1,4-비스[(3-클로로-2-히드록시프로필)아미노]안트라퀴논
noncyanide zie plating additive XD-2
2-글리시딜록시벤조니트릴
페닐글리시딜에테르
L-카르니틴염산염
L-카르니틴푸마레이트
2,3-DIHYDRO-1,4-BENZODIOXIN-2-METHANOL 4-METHYLBENZENESULFONATE
Noncyanide zinc plating additive DE
zinc plating additive DPE-1
softening agent EST
Guaiacol glycidyl ether
Metoprolol
ironicion stabilzer
에틸렌 글리콜 다이글리시딜 에테르
2-히드록시메틸-1,4-벤조디오산
2-[(1-나프틸옥시)메틸]옥시란
1,3-디요오도프로판-2-올
mnocyanide zine plating bright agent
1-(2-Hydroxy-3-sulfopropyl)-pyridinium betane
3-클로로-2-하이드록시프로필 트리메틸암모늄 클로라이드
Poly(epichlorohydrin-co-propylene oxide-co-starch)
(±)-카르니틴 하이드로클로라이드
softening agent SCI-A
noncyanide zinc plating bright additive
1-CHLORO-3-FLUOROISOPROPANOL
[[p-(2-메톡시에틸)페녹시]메틸]옥시란
N-(4-(옥시라닐메톡시)-1,2-에폭시프로판
Cross-linking agent DE
noncyanide zinc plating additive-TDAE
noncyanide zinc plating additive XD-1
이소프로필글리시딜에테르
3-AMINO-1-PHENOXY-2-PROPANOL HYDROCHLORIDE
BE-strong efficient nickel plating brightener
1,3,5-트리글리시딜 이소시아누르산염
Acetyl-L-carnitine hydrochloride
the chelating resin of crosslinking chitosan condense with salilylaldehyde
wet strength agent used for paper PAE
에피클로로하이드린 공급 업체
글로벌( 832)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3049 | 58 |
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 |
sales@yanshuochem.com | China | 101 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; |
peter68@ptchemgroup.com | China | 35425 | 58 |
| Wuxi High Mountain Hi-tech Development Co.,Ltd. | +86-86-0510-85881806 +8613357920996 |
wuxihighmountain@gmail.com | China | 67 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5251 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20203 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +8617736087130 |
catherine@yjchem.com.cn | China | 994 | 58 |
| Qingdao RENAS Polymer Material Co., Ltd. | +86-0532-86867058 +86-18562606086 |
sales@qdrenas.com | China | 1066 | 58 |
에피클로로하이드린 관련 검색:
산화프로필렌 디메틸아민 프로필렌 에틸렌옥사이드 헵타클로르 에폭시드 폴리(에피클로로히드린) (S)-에피클로로파이드린 염소(기체) 비스페놀 A-비스페놀 A 디글리시딜 에테르 중합체 에피클로로하이드린-비스페놀 A 수지 포름알데히드, (클로로메틸)옥시란과 2-메틸페놀과의 중합체 (R)-(?)-에피클로로히드린 클로로디플루오르메탄 에피클로로하이드린
Trelagliptin succinate
Gemcitabine
EPOXYPROPOXYPROPYL TERMINATED POLYDIMETHYLSILOXANE
1-Bromo-2,3-epoxypropane