Cyclopentanone
- CAS No.
- 120-92-3
- Chemical Name:
- Cyclopentanone
- Synonyms
- Cyclopentanon;ADIPIC KETONE;Cyclopentan-1-one;KETOCYCLOPENTANE;KETOPENTAMETHYLENE;Dumasin;FEMA 3910;opentanone;Adipinketon;Cyclopentsnon
- CBNumber:
- CB8709022
- Molecular Formula:
- C5H8O
- Molecular Weight:
- 84.12
- MDL Number:
- MFCD00001409
- MOL File:
- 120-92-3.mol
| Melting point | -51 °C (lit.) |
|---|---|
| Boiling point | 130-131 °C (lit.) |
| Density | 0.951 g/mL at 25 °C (lit.) |
| vapor density | 2.97 (vs air) |
| vapor pressure | 11.5 hPa (20 °C) |
| FEMA | 3910 | CYCLOPENTANONE |
| refractive index |
n |
| Flash point | 87 °F |
| storage temp. | Store below +30°C. |
| solubility | 9.18g/l slightly soluble |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Odor | Pleasant |
| explosive limit | 1.6-10.8%(V) |
| Odor Type | minty |
| Water Solubility | PRACTICALLY INSOLUBLE |
| Merck | 14,2743 |
| JECFA Number | 1101 |
| BRN | 605573 |
| Dielectric constant | 16.0(-49℃) |
| Stability | Stable. Incompatible with strong reducing agents, strong oxidizing agents, strong bases. |
| LogP | 0.7 at 25℃ |
| Substances Added to Food (formerly EAFUS) | CYCLOPENTANONE |
| CAS DataBase Reference | 120-92-3(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | 220W81TN3S |
| NIST Chemistry Reference | Cyclopentanone(120-92-3) |
| EPA Substance Registry System | Cyclopentanone (120-92-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H315-H319 | |||||||||
| Precautionary statements | P210-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10-36/38 | |||||||||
| Safety Statements | 23 | |||||||||
| RIDADR | UN 2245 3/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GY4725000 | |||||||||
| Autoignition Temperature | 445 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2914 29 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| NFPA 704 |
|
Cyclopentanone price More Price(36)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W391018 | Cyclopentanone ≥99%, FG | 120-92-3 | 1kg | $77.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W391018 | Cyclopentanone ≥99%, FG | 120-92-3 | 5kg | $343 | 2024-03-01 | Buy |
| Sigma-Aldrich | W391018 | Cyclopentanone ≥99%, FG | 120-92-3 | 10Kg | $586 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02670 | Cyclopentanone for synthesis | 120-92-3 | 2.5L | $244 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02670 | Cyclopentanone for synthesis | 120-92-3 | 250ML | $56.9 | 2023-06-20 | Buy |
Cyclopentanone Chemical Properties,Uses,Production
Physical and Chemical Properties
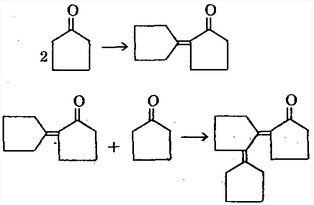
Cyclopentanone is also known Adipic Ketono. It is transparent colorless oily liquid. It has smell of special ether and also slightly mint-smell. It has a relative molecular mass being 84.12. It also has a relative density being 0.9487, melting point being-51.3 ℃, boiling point being 130.6 ℃, 23~24 ℃ (1.333 X 103Pa), the refractive index being 1.4366, and the flash point being 30 ℃. It is insoluble in water, soluble in alcohol, ether and acetone. It is narcotic at high concentrations. It can be obtained through oxidation of cyclopentanol. It can also be obtained through the heating of Adipic acid in the presence of Barium hydroxide. Cyclopentanone is also easily subject to polymerization especially in the presence of an acid. In the case of heating, it can participate in the following dehydration reactions, respectively producing 2-cyclopentylene cyclopentanone and 2'-cyclopentylene-2-cyclopentylene cyclopentanone:
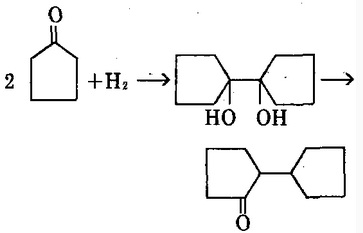
Hydrogenation can produce double-cyclopentanol with further dehydration producing 2, 2-tetramethylene cyclopentanone.
Cyclopentanone is mainly used for the manufacturing of drugs, biological agents, pesticides and rubber additives.
Aldehydes, ketones can react with diazoalkane and have the nitrogen atoms lost, generating two carbonyl compounds and epoxy compounds. When the aldehydes, ketones molecules contain electron-withdrawing groups, the reactivity is increased will boost the generation of the epoxy compound. The ketone molecule, with the increase of the hydrocarbon group, also mainly generates epoxy compound. Cyclic ketones, instead, will have ring expansion reactions. The bigger the hydrocarbyl group of the diazoalkane is, the more carbonyl compounds can be obtained.
The order of the ketone reactivity is consistent with its nucleophilic substitution order:
Cl3CCOCH3> ClCH2COCH3> CH3COCH3> CH3COC6H5> cyclohexanone> cyclopentanone> cycloheptanone> cyclooctanone.
The main purpose
1, Take the n-valeraldehyde and cyclopentanone as raw material, and first go through aldol condensation reaction and further dehydration reaction to obtain pentylene cyclopentanone, then followed by selective catalytic hydrogenation to obtain pentyl cyclopentanone. Pentyl cyclopentanone has strong floral and fruity aroma as well as nuances of jasmine. It can be used in the formulation of daily chemical flavor with the usage amount being less than 20%. IFRA has no restrictions.
2, Take n-hexanal and cyclopentanone as the raw material, first have condensation, and then perform selective hydrogenation reaction to obtain hexyl cyclopentanone. Hexyl cyclopentanone has strong jasmine smell accompanied by fruit nuances and can be used in perfume fragrance as well as the formulation of other kinds of fragrance with the usage amount being less than 5%. IFRA has no restrictions.
3, Take the 1-pentene or 1-heptene obtained by the cracking of paraffin or dehydration of the corresponding alcohol as the raw material, in the presence of t-butyl peroxide as the initiator, have addition reaction of the free radical group with cyclopentanone, generating 2-pentyl cyclopentanone (or 2-heptyl-cyclopentanone) with oxidation and ring expansion reaction to become δ-lactone (or δ-Dodecalactone).
4 Synthesis route with cyclopentanone being the starting material is of the greatest industrial production value. Cyclopentanone is first had aldol condensation reaction with n-valeraldehyde with the dehydration products further undergoing condensation and the selective hydrogenation to generate 2-pentyl cyclopentanone. It finally undergoes oxidation and ring expansion to become δ-decalactone.
5, δ-decalactone is mainly used in the formulation of edible food flavor. It is considered with the characteristic flavor of the natural cream. Before its emergence, perfumers had long been limited to application of monomer spices such as butanedione and vanillin monomers to be as the major raw material for the deployment of butter flavor. But it is generally recognized that the formulated butter flavor is worse than natural products in the respects of both taste or flavor respects. Only after the use of δ-decalactone can it have realistic cream flavor, especially in the case of using δ-Dodecalactone and δ-decalactone in combination for being as the main flavor raw material which can further improve the flavor and effect of the formulated cream spice.
6, Take cyclopentanone and pentanal as raw materials, have condensation to produce 2-(1-hydroxy) pentyl cyclopentanone which is then reacted with dimethyl malonate and hydrolyzed at 160~180 ℃, go through decarboxylation, esterification to obtain methyl dihydrojasmonate. Methyl dihydrojasmonate is the edible flavor provided by the GB2760-1996 of China for temporary application. Its aroma is better than the natural methyl jasmonate. Its property is also relatively stable.
The above information is edited by the chemicalbook of Dai Xiongfeng.
Chemical Properties
It is colorless, oily liquid with a pleasant mint smell. It has a melting point of-58.2 ℃, boiling point of 130.6 ℃, the relative density of 0.9509 (20 ℃), the refractive index of 1.4366 and the flash point of 29.82 ℃. It is miscible with ethanol, ethyl ether and slightly soluble in water. It is easy to undergo polymerization, especially in the case of trace amount of acid.
Production method
It can be obtained through the heating of Adipic acid in the presence of barium hydroxide. Mix the barium hydroxide and adipic acid uniformly and heat to 285-295 °C, further distill out the generated cyclopentanone at this temperature. The distilled calcium chloride is salted out for separating the cyclopentanone; add appropriate amount of alkaline solution to remove the adipic acid wash, then wash with water; dry with anhydrous calcium chloride; have distillation; collect the fraction in the 128-131 ℃ to obtain the finished product with the yield being 75-80%.
Category
Flammable liquid
Toxicity grading
poisoning
Acute toxicity
Intraperitoneal-mouse LD50: 1950 mg/kg; subcutaneous-Mouse LD50: 2600 mg/kg.
Data irritation
Skin-rabbit 500 mg Mild; Eyes-rabbit 100 mg severe.
Flammability and hazard characteristics
It is flammable in case of fire, high temperature and with burning producing irritated smoke.
Storage characteristics
Treasury: ventilation, low-temperature and dry; store it separately from oxidants and acids.
Extinguishing agent
Dry powder, dry sand, carbon dioxide, foam, 1211 fire extinguishing agent.
Chemical Properties
Cyclopentanone is a colorless liquid with a pleasant, slightly pepperminty odor. It is soluble in water and miscible with common organic solvents. Cyclopentanone has an agreeable, peppermint-like odor. It tends to polymerize in the presence of acids.
Occurrence
Reported found in roasted onion, baked potato, tomato, gruyere cheese, butter, heated chicken, boiled beef, heated pork, roasted pecan, yellow passion fruit juice.
Uses
Cyclopentanone is used as an intermediate in the synthesis of rubber adhesives, synthetic resins, pharmaceuticals and biologically active compounds. It acts as precursor for the preparation of cyclopentamine and also pentethylcyclanone, cyclopentobarbital. It is a useful laboratory reagent and is used as thinner for epoxies. It can also be used as a solvent in paint and varnish removers and for electronic applications. As a dry cleaning agent, it is used for oil extraction. It is also involved in the preparation of cyclopentanone derivatives like cyclopenylamine and cyclopentanol which find application in the perfume industry.
Preparation
Prepared by heating adipic acid (285 to 295°C) in the presence of barium hydroxide, distilling, ether extraction and then fractionation.
Definition
ChEBI: A cyclic ketone that consists of cyclopentane bearing a single oxo substituent.
Aroma threshold values
Aroma characteristics at 2.0%: musty, slightly toasted bitter almondlike nutty, solventlike with a powdery nuance.
Taste threshold values
Taste characteristics at 20 ppm: musty, toasted nutty with a slight meaty nuance.
General Description
A clear colorless liquid with a petroleum-like odor. Flash point 87°F. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Cyclopentanone polymerizes easily, especially in the presence of acids. Can react with oxidizing materials, i.e. hydrogen peroxide.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Safety Profile
Moderately toxic by intraperitoneal and subcutaneous routes. A skin and severe eye irritant. Dangerous fire hazard when exposed to heat or flame; can react with oxidizers. To fight fire, use alcohol foam, foam, CO2, dry chemical. Potentially explosive reaction with hydrogen peroxide + nitric acid. When heated to decomposition it emits acrid smoke and fumes. See also KETONES.
Purification Methods
Shake it with aqueous KMnO4 to remove materials absorbing around 230 to 240nm. Dry it with Linde-type 13X molecular sieves and fractionally distil it. It has also been purified by conversion to the NaHSO3 adduct which, after crystallising four times from EtOH/water (4:1), is decomposed by adding to an equal weight of Na2CO3 in hot H2O. The free cyclopentanone is steam distilled from the solution. The distillate is saturated with NaCl and extracted with *benzene which is then dried (anhydrous K2CO3) and evaporated. The residue is then distilled [Allen, et al. J Chem Soc 1909 1960]. [Beilstein 7 IV 5.]
Cyclopentanone Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8817 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18644 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 299 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21638 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9220 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29885 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
View Lastest Price from Cyclopentanone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-01 | Cyclopentanone
120-92-3
|
US $10.00 / kg | 1kg | 99% | 100 TON | Hebei Weibang Biotechnology Co., Ltd | |
 |
2024-10-11 | Cyclopentanone
120-92-3
|
US $10.70 / kg | 10kg | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-01-08 | Cyclopentanone
120-92-3
|
US $0.00-0.00 / KG | 10KG | 99% | 50000tons | Wuhan Boyuan Import & Export Co., LTD |
-

- Cyclopentanone
120-92-3
- US $10.00 / kg
- 99%
- Hebei Weibang Biotechnology Co., Ltd
-

- Cyclopentanone
120-92-3
- US $10.70 / kg
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Cyclopentanone
120-92-3
- US $0.00-0.00 / KG
- 99%
- Wuhan Boyuan Import & Export Co., LTD
120-92-3(Cyclopentanone)Related Search:
1of4





