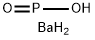barium phosphinate
- CAS No.
- 14871-79-5
- Chemical Name:
- barium phosphinate
- Synonyms
- barium phosphinate;Barium bisphosphinate
- CBNumber:
- CB8919720
- Molecular Formula:
- BaH4O4P2
- Molecular Weight:
- 267.303882
- MDL Number:
- MFCD00799189
- MOL File:
- 14871-79-5.mol
| Melting point | decomposes at 100–150℃ [CRC10] |
|---|---|
| Density | 2.900 |
| vapor pressure | 0Pa at 25℃ |
| solubility | soluble in H2O; insoluble in ethanol |
| form | monoclinic plates |
| color | Colorless |
| Water Solubility | g/100mL: 28.6 (17°C), 33.3 (100°C); insoluble alcohol [MER06] |
| FDA UNII | DC9518W9VC |
| EPA Substance Registry System | Phosphinic acid, barium salt (14871-79-5) |
barium phosphinate Chemical Properties,Uses,Production
Chemical Properties
monoclinic platelets; can be prepared by reacting white phosphorus and barium hydroxide; used in nickel plating; anhydrous material, Ba(H2PO2)2, is a white, odorless, crystal(s) powder(s) [MER06] [HAW93]
Production Methods
Barium hypophosphite and PH3 are produced in the reaction of white phosphorus with Ba(OH)3. A solution of 120 g of crystalline Ba(OH) in 1200 ml of water is heated for about four hours with 30 g of white P in a round bottom flask. The flask is provided with a long glass tube extending to the stack of the exhaust hood to conduct away the spontaneously igniting phosphine mixture. When the P is completely dissolved, CO2 is introduced to precipitate the excess Ba(OH)3. The precipitate is filtered off and washed with hot water. The solution and the wash water are combined and evaporated to half the original volume, refiltered, and evaporated until crystallization begins. Some alcohol is then added, and the mixture is left to cool. The resulting crystals are suction-filtered, and the mother liquor is again evaporated until crystallization takes place. The accumulated salts are combined and recrystallized from hot water to produce barium phosphinate. The yield is 40-60 g.
Flammability and Explosibility
Not classified
Purification Methods
It precipitates from aqueous solution (3mL/g) on adding EtOH. Its solubility in H2O is 28.6% at 17o and 33.3% at 100o. [Klement in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 557 1963.]
barium phosphinate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-87576359 15353716720 | 1039@dideu.com | China | 10009 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| Shaanxi Dideu Newmaterial Co., Ltd. | 58 |