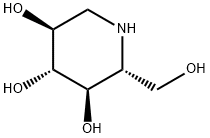
1-Deoxynojirimycin
- CAS No.
- 19130-96-2
- Chemical Name:
- 1-Deoxynojirimycin
- Synonyms
- DNJ;moranoline;DEOXYNOJIRIMYCIN;Sepetaprost;Duvoglustat;DNM;S-GI;moranolin;bay-h5595;uvoglusta
- CBNumber:
- CB9128150
- Molecular Formula:
- C6H13NO4
- Molecular Weight:
- 163.17
- MDL Number:
- MFCD00063474
- MOL File:
- 19130-96-2.mol
- MSDS File:
- SDS
| Melting point | 195-196°C |
|---|---|
| Boiling point | 361.1±42.0 °C(Predicted) |
| Density | 1.456±0.06 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| RTECS | TN4350300 |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in Water up to 25 mg/ml). |
| pka | 13.77±0.70(Predicted) |
| form | Powder |
| color | White |
| Water Solubility | Soluble in water, dimethyl sulfoxide and methanol. |
| BRN | 3588039 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. |
| LogP | -1.8 |
| CAS DataBase Reference | 19130-96-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | FZ56898FLE |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P271-P280 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| HS Code | 29329990 | |||||||||
| NFPA 704 |
|
1-Deoxynojirimycin price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 08012 | 1-Deoxynojirimycin analytical standard | 19130-96-2 | 10mg | $623 | 2024-03-01 | Buy |
| Sigma-Aldrich | SAB5300111 | Monoclonal Anti-DNM1 antibody produced in mouse clone 3G4B6, ascites fluid | 19130-96-2 | 100μL | $508 | 2022-05-15 | Buy |
| Alfa Aesar | J62602 | (+)-1-Deoxynojirimycin | 19130-96-2 | 100mg | $563 | 2024-03-01 | Buy |
| Cayman Chemical | 21500 | 1-Deoxynojirimycin ≥95% | 19130-96-2 | 5mg | $62 | 2024-03-01 | Buy |
| Cayman Chemical | 21500 | 1-Deoxynojirimycin ≥95% | 19130-96-2 | 10mg | $109 | 2024-03-01 | Buy |
1-Deoxynojirimycin Chemical Properties,Uses,Production
Chemical Name
(2R,3R,4R,5S)-2-Hydroxymethyl-piperidine-3,4,5-triol
Alkaloids
1-deoxynojirimycin is referred DNJ for short , it is an alkaloid extracted from the bark of mulberry leaves and roots, but it also exists in other plants and microorganisms. This product is an effective α-glucosidase inhibitor, it has significant hypoglycemic effect. After 1-deoxynojirimycin goes into the human body, it can inhibit sucrose, maltase, α-glucosidase enzyme, α-amylase decomposing starch, sugar in the human body , thereby it can block the body's absorption of sugar, inhibiting blood sugar rising to achieve the effect of prevention and treatment of diabetes, the use of it does not cause changes in diet . In addition, DNJ can inhibit glucose modification process of HIV tunica glycoprotein , at the same time, the accumulation of immature glycoproteins may inhibit cell fusion, viral and host cell receptor can combine,which causes syncytia formation to inhibit the replication of MoLV ,then the virus activity is inhibited.
Nojirimycin is first discovered from Streptomyces, and natural DNJ is first isolated from the bark of mulberry root. In plants, from mulberry, dayflower, hyacinth and Adenophora plants, DNJ has been isolated and identified , DNJ has the highest content in the mulberry and because of mulberry varieties, medicinal parts, seasonal climate, geography, soil, leaf position, different developmental stages and other factors , there is a big difference. In a microorganism, from a variety of Streptomyces and Bacillus,DNJ is isolated ,it is also found that two kinds of endophytes separated from Mulberry including Stenotrophomonas oligotrophic Pseudomonas and Micrococcus can produce DNJ,fermentation conditions of a variety of microbial production of DNJ are studied. In insects, in addition to silkworm rich in DNJ , single or oligophagous insects with eating mulberry leaves habit including wild silkworm, mulberry geometrid, Diaphania pyloalis Walker , mulberry white capterpillar are also rich in DNJ , DNJ in insects bodies are from the food , content of DNJ in Bombyx bodies is different due to the different varieties of silkworm, developmental stages, tissues and organs as well as feed and other factors, with the silkworm age of progress ,there is the existence of cyclical changes in absorption and accumulation and excretion of DNJ. Now DNJ biosynthetic pathways in Streptomyces, Bacillus and Commelina bodies are explored and it is found that synthesis of DNJ has different mechanisms in different species . In addition, three main synthesis methods of 1-deoxynojirimycin are proven , some of the synthetic derivatives of DNJ have been used clinically.
Recent studies show that the active ingredient of mulberry DNJ (l-deoxynojirimycin), only exists in mulberry leaves , by blocking the α-glucosidase enzymes to hinder sugar becoming to glucose, mulberry leaf extract can inhibit intestinal glucose absorption. This can suppress the blood sugar level and blood pressure rising , and it can have good inhibitory effect on variability of imidazopyridine, benzopyrene and other carcinogenic substances,it has anti-cancer effect, at the same time ,mulberry leaf extract can reduce cholesterol, and improve liver function and eliminate constipation and so on.
Detection method
After extraction, 1-Deoxynojirimycin (DNJ) contents can be determined by HPLC using various detectors such as fluorescent, evaporative light-scattering detectors, pulsed amperometric, and Mass spectrometers. Since the DNJ molecule does not contain a chromophore, the quantification of DNJ through spectral analysis needs derivatization, i.e., an analytical approach for the quantification of nitrogen-containing compounds. To quantify and resolve structural information of DNJ by GC-MS, Bajpai and Rao used trimethylsilyl (TMS) derivatization.
Description
Deoxynojirimycin (19130-96-2) inhibits α-glucosidase I and II.1,2 Inhibits human immunodeficiency virus envelope glycoprotein-mediated membrane fusion at the CXCR4 binding step.3 May be used to produce an affinity ligand for purifying glucosidase 1.4 Deoxynojirimycin was used to inhibit ER glucosidases I and II allowing for the discovery of a second mechanism for deglucosylation of N-linked oligosaccharides in PhaR1.7, a mouse lymphoma cell line.5
Chemical Properties
White Crystalline Solid
Occurrence
1-Deoxynojirimycin (DNJ), a product of fermentation, exists in mulberry leaves and Commelina communis (dayflower) as well as is isolated from several bacterial strains such as Bacillus and Streptomyces species. Mulberry, a common deciduous plant, belongs to the genus Morus (Moraceae family). Numerous researchers have reported the DJN contents in different parts and various varieties of mulberry. Various mulberry varieties contain DNJ contents ranging between 0.68 and 2.78 mg/gm, while this fraction varies between 1.57 and 3.48 mg/gm in different Chinese mulberry leaves. Moreover, mulberry shoots contain the highest contents of DNJ, followed by young mulberry leaves. Mature leaves of mulberry contain the least content of DNJ. All the mulberry leave groups showed a 1–2-fold increase in DNJ contents compared to unfermented mulberry leaf powder extract (UFMLE). Additionally, fermented mulberry leaf powder extract exhibited higher α-glucosidase activity than UFMLE. DNJ-rich food products can be prepared by fermenting mulberry leaves using the above-mentioned fermenting agents[6].
Uses
Deoxynojirimycin inhibits mammalian glucosidase 1. As well, it inhibits intestinal and lysosmal alpha-glucosidases, beta-glucosidase from sweet almonds, pancreatic alpha-amylase and amyloglucosidase.
Uses
An alpha-glucosidase inhibitor. Interferes with normal processing of N-linked glycoproteins.(+)-1-Deoxynojirimycin acts as an inhibitor of alfa-glucosidase I and II and maltase-glucoamylase. It also inhibits mammalian glucosidase, intestinal and lysosmal, beta-glucosidase from sweet almonds, pancreatic alfa-amylase and amyloglucosidase. Further, it serves as a enzyme enhancer for the treatment of Fabry and Pompe disease.
Uses
An inhibitor of α-glucosidase I and II
Definition
ChEBI: An optically active form of 2-(hydroxymethyl)piperidine-3,4,5-triol having 2R,3R,4R,5S-configuration.
Biological Activity
Inhibitor of glucosidase I (K i = 2.1 mM) and II (K i = 7 mM).
storage
+4°C
References
1) Fuhrmann et al. (1985), Inhibitors of oligosaccharide processing; Biochim. Biophys, Acta, 825 95
2) Hughs and Rudge (1994), Deoxynojirimycin: synthesis and biological activity; Nat. Prod. Rep., 11 135
3) Papandreou et al. (2002), The alpha-glucosidase inhibitor 1-deoxynojirimycin blocks human immunodeficiency virus envelope glycoprotein-mediated membrane fusion at the CXCR4 binding step; Mol. Pharmacol., 61 186
4) Hettkamp et al. (1984), Purification by affinity chromatography of glucosidase I, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligosaccharides; Eur. J. Biochem., 142 85
5) Suh et al. (1992), Identification of a novel mechanism for the removal of glucose residues from high mannose-type oligosaccharides; J. Biol. Chem., 267 21671.
[6] Kuo Gao. “1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing.” Molecules 21 1 (2016).
1-Deoxynojirimycin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3772 | 58 |
| Hengshui Haoye Co.,Ltd. | +86-2102300 +86-18632882519 | hy@haoyecom.cn | China | 299 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 390 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18775 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2986 | 55 |
View Lastest Price from 1-Deoxynojirimycin manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-28 | 1-Deoxynojirimycin
19130-96-2
|
US $0.00 / kg | 1kg | 1%-5% 10% 15% | 1000 kg | BINBO BIOLOGICAL CO.,LTD | |
 |
2024-11-28 | 1-Deoxynojirimycin; DNJ
19130-96-2
|
US $0.00 / MG | 20MG | 98% HPLC | 1000KG | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-11-28 | 1-Deoxynojirimycin; 1-DNJ
19130-96-2
|
US $0.00 / KG | 1KG | ≥98% HPLC | 1000KG | Changsha Staherb Natural Ingredients Co., Ltd. |
-

- 1-Deoxynojirimycin
19130-96-2
- US $0.00 / kg
- 1%-5% 10% 15%
- BINBO BIOLOGICAL CO.,LTD
-

- 1-Deoxynojirimycin; DNJ
19130-96-2
- US $0.00 / MG
- 98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- 1-Deoxynojirimycin; 1-DNJ
19130-96-2
- US $0.00 / KG
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
19130-96-2(1-Deoxynojirimycin)Related Search:
1of4