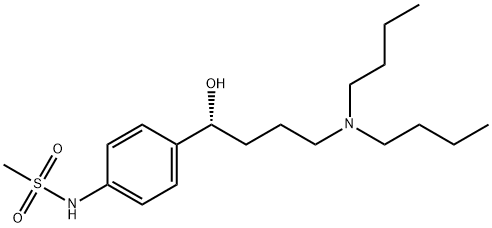
artilide
- CAS No.
- 133267-19-3
- Chemical Name:
- artilide
- Synonyms
- artilide;Methanesulfonamide, N-[4-[(1R)-4-(dibutylamino)-1-hydroxybutyl]phenyl]-
- CBNumber:
- CB91323835
- Molecular Formula:
- C19H34N2O3S
- Molecular Weight:
- 370.55
- MDL Number:
- MOL File:
- 133267-19-3.mol
| FDA UNII | H5L34MU3TQ |
|---|
artilide Chemical Properties,Uses,Production
Originator
Artilide fumarate,Upjohn (Pharmacia)
Uses
Cardiac depressant (anti-arrhythmic).
Uses
Artilide is used as a pharmaceutical antiarrhythmic agent.
Definition
ChEBI: Artilide is an organic amino compound and a member of benzenes.
Manufacturing Process
A mechanically stirred solution of aniline (139.7 g, 1.5 mol) in pyridine (2 L),
under N2 is cooled in an icebath. Methanesulfonyl chloride (171.8 g, 1.5 mol)
is added dropwise to this solution while the temperature is maintained at 15°-
20°C, which results in a red-orange color change in the reaction mixture.
After the addition is complete the ice bath is removed and the reaction is
allowed to continue at room temperature for 2.5 h. The reaction mixture is
concentrated in vacuo and the residue is combined with 700 ml of water which
results in crystallization of a dark red material. This material is filtered and
washed several times with water. The filtered material is dissolved in CH2Cl2,
washed with brine, dried (Na2SO4), and concentrated in vacuo to give four
crops (157.37 g, 19.27 g, 26.55 g, 5.07 g) of methanesulfonanilide, melting
point 93°C (decolorizing with Darco carbon and crystallization from ethyl
acetate).
A mechanically stirred suspension of aluminum chloride (88.0 g, 0.66 mol)
and 150 ml of carbon disulfide under N2 is cooled in an ice bath.
Methanesulfonanilide (30.0 g, 0.175 mol) and succinic anhydride (17.5 g,
0.175 mol) are combined and added rapidly to the cooled reaction mixture.
The ice bath is removed and the mixture is stirred at room temperature for 6
h.
The reaction mixture is then heated to 55°C and allowed to continue for 18 h.
The reaction mixture is separated into two layers the bottom of which
solidifies. The upper layer is decanted and the remaining solid layer is
decomposed with ice. The resulting suspension is filtered and the solid is
washed several times with methylene chloride and dissolved in a mixture of
saturated sodium bicarbonate (500 ml) and water (500 ml). This solution is acidified (pH 2) with HCl and the resulting precipitate is collected by filtration,
redissolved in NaHCO3 and reprecipitated with HCl. The solid is collected by
filtration and dried to give 4-[(methylsulfonyl)amino]-γ-oxobenzenebutanoic
acid, melting point 198°-200°C.
A mixture of 4'-[(methylsulfonyl)amino]-γ-oxobenzenebutanoic acid and 1-
hydroxybenzotriazole in dimethylformamide (DMF) under nitrogen, is treated
with dibutylamine in DMF. The mixture is cooled in an ice bath and 1-ethyl-3-
(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) added in portions
over 5 min. The mixture is stirred in the cold 1 h and overnight at room
temperature. The solvent is removed in vacuo (bath temperature 35°C). The
residue is treated with ice and ethyl acetate and the organic layer washed
sequentially with 0.5 N monopotassium sulfate, cold 4% NaHCO3, cold water
and finally brine. The organic solution is dried (Na2SO4) and concentrated in
vacuo. The N,N-dibutyl-γ-oxo-4-[(methylsulfonyl)amino]benzenebutan-amide
(crystallized from ethyl acetate-hexane) is obtained.
Lithium aluminum hydride is suspended in dry tetrahydrofuran (THF), under
nitrogen and the mixture cooled in an ice bath. To this mixture is added N,Ndibutyl-
γ-oxo-4-[(methylsulfonyl)amino]benzenebutanamide, (partly as a
suspension in THF added over 10 min and partly as a solid added in portions
over 30 min).
The mixture is stirred for 2.5 h in the cold. The cold reaction mixture is then
treated cautiously with a saturated solution of sodium potassium tartrate in
water and stirred for 10 min in the cold. This mixture is extracted with EtOAc.
The pooled ethyl acetate extracts are washed with brine, dried (Na2SO4) and
concentrated in vacuo to give a solid. The aqueous residue from the above
extractions is diluted with 10 ml of water and extracted with ethyl acetate.
The pooled extract is washed with brine, dried (Na2SO4) and concentrated to
give solid. The two solids are recrystallized separately from EtOAc to give N-4-
[4-(dibutylamino)-1-hydroxybutyl]phenyl]methanesulfonamide (recrystallized
from EtOAc-hexane).
By recrystallyzation of stereo-isomers of N-4-[4-(dibutylamino)-1-
hydroxybutyl]phenyl]methanesulfonamide may be obtained the (R)-N-4-[4-
(dibutylamino)-1-hydroxybutyl]phenyl]methanesulfonamide, melting point
179°-180°C.
Therapeutic Function
Antiarrhythmi