GOLD
- CAS No.
- 7440-57-5
- Chemical Name:
- GOLD
- Synonyms
- Au;GC;GOLD POWDER;COLLOIDAL GOLD;GOLD STANDARD SOLUTION;Glod;ci77480;GOLD SOL;Gold wire;Gold leaf
- CBNumber:
- CB9169816
- Molecular Formula:
- Au
Lewis structure

- Molecular Weight:
- 196.97
- MDL Number:
- MFCD00003436
- MOL File:
- 7440-57-5.mol
- MSDS File:
- SDS
| Melting point | 1063 °C (lit.) |
|---|---|
| Boiling point | 2808 °C (lit.) |
| Density | 19.3 g/mL at 25 °C (lit.) |
| refractive index |
n |
| Flash point | 4 °C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | wire |
| color | purple |
| Specific Gravity | 19.3 |
| PH | 6-8 |
| PH Range | 6 - 8 |
| Resistivity | 2.05 μΩ-cm, 0°C |
| Water Solubility | Soluble in hot sulfuric acid and aqua regia. Insoluble in water and acid. |
| Sensitive | Light Sensitive |
| λmax | 980 nm |
| Merck | 13,4529 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability | Stable. May react with halogens, strong oxidizing agents, ammonia, hydrogen peroxide. Reaction with ammonia or hydrogen peroxide may form explosive materials. |
| CAS DataBase Reference | 7440-57-5(CAS DataBase Reference) |
| EWG's Food Scores | 1-4 |
| FDA UNII | 79Y1949PYO |
| EPA Substance Registry System | Gold (7440-57-5) |
| Absorption | 1 at 523nm |
| UNSPSC Code | 41116107 |
| NACRES | NA.26 |
| Modulus of Elasticity | 77.2 GPa, 60% Cold Worked |
|---|---|
| Poissons Ratio | 0.42 |
| Shear Modulus | 27.2 GPa, 60% Cold Worked; Calculated Value |
| Hardness, Vickers | 25 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H412 | |||||||||
| Precautionary statements | P273-P501 | |||||||||
| Hazard Codes | Xi,Xn,F,C | |||||||||
| Risk Statements | 36/38-43-67-65-63-48/20-38-11-34-23-52/53 | |||||||||
| Safety Statements | 26-36/37/39-45-62-36/37-61-23 | |||||||||
| RIDADR | UN 1789 8/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MD5420000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 3822 00 00 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 7440-57-5(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
GOLD price More Price(887)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | GF99590585 | Gold foil, thickness 0.007?mm, diameter 15 mm, purity 99.9% | 7440-57-5 | 10EA | $2990 | 2024-03-01 | Buy |
| Sigma-Aldrich | GF94801002 | Gold foil, thickness 0.250?mm, size 12.5 × 12.5?mm, high purity 99.99% | 7440-57-5 | 1EA | $679 | 2024-03-01 | Buy |
| Sigma-Aldrich | GF96608245 | Gold wire, 0.05?mm diameter, length 5 m, purity 99.99+% | 7440-57-5 | 1EA | $449 | 2024-03-01 | Buy |
| Sigma-Aldrich | GF94801002 | Gold foil, thickness 0.250?mm, size 12.5 × 12.5?mm, high purity 99.99% | 7440-57-5 | 5EA | $2750 | 2024-03-01 | Buy |
| Sigma-Aldrich | GF93517386 | Gold wire, 0.125?mm diameter, length 0.2 m, high purity 99.99+% | 7440-57-5 | 1EA | $210 | 2024-03-01 | Buy |
GOLD Chemical Properties,Uses,Production
Description
Metallic gold is virtually insoluble, except in aqua regia. Gold exists in three primary forms, elemental, Au (I), and Au (III). As a precious metal, it is resistant to ionization and generally considered biologically benign in its elemental state.

Gold is not permitted for use in foods, drugs or cosmetics as a coloring agent in the U.S. However, surface decoration of foods under conditions which preclude consumption is not considered to be a food use and is, therefore, exempt from regulations concerning food use. The use of gold in the decoration of food is more popular in Europe, where, probably as the result of its limited use, its use has been accepted.
Chemical Properties
Gold does not have a distinctive odor at room temperature, but when heated it emits a sweet odor that is detected with difficulty. Chloroauric acid composed of yellow-orange crystalline powder has a faint chlorine odor.
Physical properties
Gold is a soft, malleable, ductile, dense metal with a distinctive yellow color. It is almost aheavy as lead, and both can be cut with a knife. One ounce of gold can be beaten and poundedinto a thin sheet that is only a few molecules thick and that will cover over 300 square feetof surface. Although gold is chemically nonreactive, it will react with chlorine and cyanidesolutions and can be dissolved in aqua regia. Its melting point is 1,064.4°C, its boiling pointis 2,808°C, and its density is 19.3 g/cm3 (as compared to lead’s density of 11.35 g/cm3).
Isotopes
There are a total of 54 isotopes of gold, only one of which is stable: Au-197,which accounts for the element’s total natural existence on Earth. The remaining 53 isotopesare radioactive, are artificially produced in nuclear reactors or particle accelerators,and have half-lives ranging from a few microseconds to a few seconds to a few hours toa few days.
Origin of Name
The name “gold” is Anglo-Saxon as well as from the Sanskrit word javal. The symbol Au is from the Latin word aurum, which means “shining dawn.”
Occurrence
Gold is the 72nd most abundant element and is widely spread around the world, but it is not evenly distributed through the surface of the Earth. It is usually found in a few concentrated regions, sometimes in pure flake and nugget metallic forms. Most of it exists in conjunction with silver ore, quartz (SiO2), and the ores of tellurium, zinc, and copper. About one milligram of gold exists in every ton of seawater (this is about 10 parts of gold per trillion parts of seawater, which amounts to a total of about 79 million tons of gold in solution). No economical method of extracting gold from seawater has been developed to recover this treasury of the sea.
Free metallic gold is found in veins of rocks and in ores of other metals. Alluvial gold (placer deposits) is found in the sand and in the gravel at the bottom of streams where it has been deposited as a result of the movement of water over eons. Most gold is recovered from quartz veins called loads and from ores that are crushed.
Characteristics
Gold is not only pleasing to look at but also pleasing to touch, which made it a desirablemetal for human decoration in prehistoric days. It is still the preferred metal for jewelry makingtoday.
Gold is classed as a heavy, noble metal located just below copper and silver in group 11 ofthe periodic table. Gold is a good conductor of electricity as well as an excellent heat reflectorof infrared radiation, which makes it an efficient thin coating on glass in skyscrapers to reflectthe heat of sunlight.
The purity of gold is measured in “carats” (one carat is equal to one part in twenty-four). Thepurest gold is rated at 24 carats, but it is much too soft to be used for jewelry. Good jewelry ismade from 18-carat gold that is 18 parts gold and six parts alloy metal. Thus, an 18-carat goldring is about 75% pure gold and contains about 25% of another metal, such as nickel or copper,to make it harder and more durable. Other alloy metals mixed with gold are silver, platinum, andpalladium—all used to increase gold’s strength and reduce its cost. Some less expensive jewelrycontains 14 or 10 carats of gold (14/24 or 10/24) as well as some other alloy metals.
History
Known and highly valued from earliest times, gold is found in nature as the free metal and in tellurides; it is very widely distributed and is almost always associated with quartz or pyrite. It occurs in veins and alluvial deposits, and is often separated from rocks and other minerals by sluicing and panning operations. About 25% of the world’s gold output comes from South Africa, and about two thirds of the total U.S. production now comes from South Dakota and Nevada. The metal is recovered from its ores by cyaniding, amalgamating, and smelting processes. Refining is also frequently done by electrolysis. Gold occurs in sea water to the extent of 0.1 to 2 mg/ton, depending on the location where the sample is taken. As yet, no method has been found for recovering gold from sea water profitably. It is estimated that all the gold in the world, so far refined, could be placed in a single cube 60 ft on a side. Of all the elements, gold in its pure state is undoubtedly the most beautiful. It is metallic, having a yellow color when in a mass, but when finely divided it may be black, ruby, or purple. The Purple of Cassius is a delicate test for auric gold. It is the most malleable and ductile metal; 1 oz. of gold can be beaten out to 300 ft2. It is a soft metal and is usually alloyed to give it more strength. It is a good conductor of heat and electricity, and is unaffected by air and most reagents. It is used in coinage and is a standard for monetary systems in many countries. It is also extensively used for jewelry, decoration, dental work, and for plating. It is used for coating certain space satellites, as it is a good reflector of infrared and is inert. Gold, like other precious metals, is measured in troy weight; when alloyed with other metals, the term carat is used to express the amount of gold present, 24 carats being pure gold. For many years the value of gold was set by the U.S. at $20.67/troy ounce; in 1934 this value was fixed by law at $35.00/troy ounce, 9/10th fine. On March 17, 1968, because of a gold crisis, a two-tiered pricing system was established whereby gold was still used to settle international accounts at the old $35.00/troy ounce price while the price of gold on the private market would be allowed to fluctuate. Since this time, the price of gold on the free market has fluctuated widely. The price of gold on the free market reached a price of $620/troy oz. in January 1980. More recently, the U.K. and other nations, including the I.M.F. have sold or threatened to sell a sizeable portion of their gold reserves. This has caused wide fluctuations in the price of gold. Because this has damaged the economy of some countries, a moratorium for a few years has been declared. This has tended to stabilize temporarily the price of gold. The most common gold compounds are auric chloride (AuCl3) and chlorauric acid (HAuCl4), the latter being used in photography for toning the silver image. Gold has forty-eight recognized isotopes and isomers; 198Au, with a half-life of 2.7 days, is used for treating cancer and other diseases. Disodium aurothiomalate is administered intramuscularly as a treatment for arthritis. A mixture of one part nitric acid with three of hydrochloric acid is called aqua regia (because it dissolved gold, the King of Metals). Gold is available commercially with a purity of 99.999+%. For many years the temperature assigned to the freezing point of gold has been 1063.0°C; this has served as a calibration point for the International Temperature Scales (ITS-27 and ITS-48) and the International Practical Temperature Scale (IPTS-48). In 1968, a new International Practical Temperature Scale (IPTS68) was adopted, which demanded that the freezing point of gold be changed to 1064.43°C. In 1990 a new International Temperature Scale (ITS-90) was adopted bringing the t.p.(triple point) of H2O (t90 (°C)) to 0.01°C and the freezing point of gold to 1064.18°C. The specific gravity of gold has been found to vary considerably depending on temperature, how the metal is precipitated, and cold-worked. As of December 2001, gold was priced at about $275/troy oz. ($8.50/g).
Uses
Gold’s chemical and physical properties make it a very versatile element. Its noncorrosivenature provides protection as plating for other metals. Its malleability and ductile qualities meanit can be formed into many shapes, including very thin sheets (gold leaf) and very thin golddiode wires. Gold has the ability to carry electricity with little resistance, making it an excellentcomponent for all kinds of electronic equipment. Gold leaf finds many uses in surgery,space vehicles, and works of art. Gold electronic switches do not create a dangerous spark whenengaged, and they last for a long time. The element’s reflective surface provides protection frominfrared heat radiation as a coating on the visors of aerospace personnel, as well as on large windowexpanses in buildings. Its color and durability make it a major metal for the jewelry industry.Gold has been used to replace teeth for many ages, and the teeth usually last longer than theperson wearing them. Gold is also the worldwide monetary standard, although the United Statesabandoned the gold standard in the 1930s. Even so, gold is still traded as a commodity. Smallamounts of other metals are added to gold coinage for hardening purposes so that the coins willnot wear out with use. Gold bars (bullion) are stored in the treasuries of most countries. Somecountries maintain huge stockpiles of gold for both monetary and industrial uses.
Two forms of gold provide medical treatments. The radioactive isotope Au-198, with a shorthalf-life of 2.7 days, is used to treat cancer and is produced by subjecting pure gold to neutronswithin a nuclear reactor.
Uses
Among the first uses of gold are religious and cultural, as it became many decorations and idols for thousands of years. The next major use of gold was as the first monetary systems, representing wealth and gradually replacing the barter system until the advent of modern economics. Gold has found many industrial uses because of its excellent electrical and thermal conductivity properties. It is used for plating other metals and as an alloying metal. It is used in themanufacture of jewelry, dental inlays, art, currency, and electronic components, and in some medical devices to provide radio opacity. Gold compounds have also found use in medicine in the treatment of certain cancers, rheumatoid arthritis, and discoid lupus (a rare skin disease), and in specialized surgical procedures.
Uses
In manufacture of jewelry; in gold plating other metals; as a standard of currency; most frequently alloyed with silver and copper. For use in medicine, see Gold, Radioactive, Colloidal.
Gold has excellent properties as a reflecting mirror. Gold black is utilized as the absorber of light by
depositing on the plane of light incidence of a thermocouple.
Definition
A transition metal that occurs native. It is unreactive and is very ductile and malleable. Gold is used in jewelry, often alloyed with copper, and in electronics and colored glass. Pure gold is 24 carat; 9 carat indicates that 9 parts in 24 consist of gold. Symbol: Au; m.p. 1064.43°C; b.p. 2807°C; r.d. 19.320 (20°C); p.n. 79; r.a.m. 196.96654.
Production Methods
Gold has been known to mankind for more than 4000 years
and since the times of the ancient Egyptian, Babylonian,
Roman, Greek, and Chinese empires, gold has been recognized as the king of metals and an object of prestige
and value. Icons, objects of religious significance, coinage,
jewelry, and funerary artifacts provide evidence of the goldsmith’s
craft from early times.
During the Middle Ages, the demand for gold led the early
alchemists to attempt converting base metals into gold.
Chinese alchemy was associated with Taoist philosophy
that purported to transmute base metals into gold by use
of a “medicine”; the gold produced was held to have amazing
medicinal power to cure all diseases and to prolong life.
Early Egyptians and Greeks including Aristotle were masters
of applied chemistry, although at the time it was closely
linked with the embalming of bodies and religious rituals.
Much of the early chemistry of metals was shrouded in
superstition, astrological speculations, and obscure symbolism.
Although transmutation of lead or iron to gold
was thought possible in Arabic science up to 1000 A.D.,
the philosophy declined in the eleventh and twelfth centuries
as more rational scientific ideas emerged and the true science
of chemistry became established.
Gold was among the first metals to be mined. It occurs
naturally in its metallic form in many parts of the world and is
distinctive by its lustrous appearance. In its elementary state,
gold is found admixed with quartz and pyrite in alluvial
deposits in rivers and streams in the form of small scales or
nuggets that were avidly sought in the days of the gold rushes
as in California, Klondike (1890s), and Fraser
Canyon (1850s). Ores containing gold are quite rare and
mostly comprise gold telluride (calaverite, krennerite—
AuTe2) and complexes such as sylvanite, nagyagite, petzite,
and krennerite with silver, antimony, and lead. Gold possibly
comprises no more than 0.004 ppm of the Earth’s crust but
has a worldwide distribution with major deposits in South
Africa, Siberia, China, South and North America, India, and
Australia.
Definition
Metallic element of atomic number 79, Group IB of the periodic table, aw 196.9665, valences of 1, 3; no stable isotopes.
Definition
gold: Symbol Au. A soft yellow malleablemetallic transition element;a.n. 79; r.a.m. 196.967; r.d. 19.32;m.p. 1064.43°C; b.p. 2807±2°C. Goldhas a face-centred-cubic crystal structure.It is found as the free metal ingravel or in quartz veins, and is alsopresent in some lead and copper sulphideores. It also occurs combinedwith silver in the telluride sylvanite,(Ag,Au)Te2. It is used in jewellery,dentistry, and electronic devices.Chemically, it is unreactive, beingunaffected by oxygen. It reacts withchlorine at 200°C to form gold(III)chloride. It forms a number of complexeswith gold in the +1 and +3 oxidationstates.
Reactions
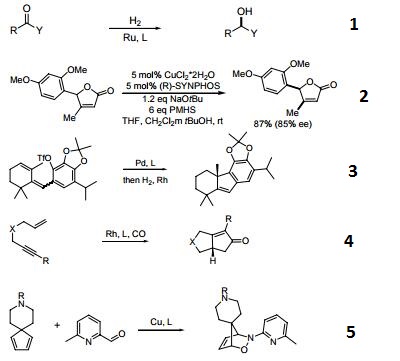
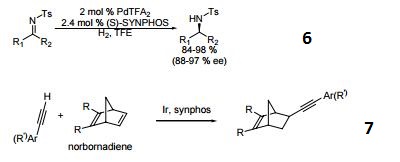
- A chiral diphosphine ligand used in the highly-enantioselective hydrogenation of ketoesters, hydroxyketones, ketophosphonates and succinates.
- A ligand used for the dynamic kinetic resolution of α,β−unsaturated lactones via asymmetric copper-catalyzed conjugate reduction.
- Used in the intramolecular Heck reaction for the synthesis of diterpenoids.
- Used in asymmetric Pauson-Khand reaction.
- Used in asymmetric iminonitroso Diels-Alder reaction.
- Palladium catalyzed asymmetric hydrogenation of N-tosyl ketimines.
- Ligand for asymmetric hydroalkynylation of norbornadienes
General Description
Gold nanoparticles PEG 5000 biotin terminated.The preparation of biotinylated gold nanoparticles involves two surface modification steps. First, the carboxyl-terminated alkanethiol is attached to the surface of gold nanoparticles via chemisorption in the presence of a stabilizing agent. This step is followed by the reaction of the carboxyl groups with (+)-biotinyl-3,6,9,-trioxaundecanediamine and 2-(2-aminoethoxy)ethanol.
Hazard
Pure gold, if ingested, can cause skin rash or even a sloughing off of skin. It can also causekidney damage and problems with the formation of white blood cells.
Pharmaceutical Applications
Gold has a long-standing tradition in medicine, as it has been used by many nations for thousands of years. From as early as 2500 BC, Arabians, Chinese and Indians used gold compounds for medicinal purposes. In mediaeval times, the elixir aurum potabile , whichwas an alcoholic mixture of herbs with some gold flakes, was sold by medicine men travelling around Europe and this elixir was supposed to cure most diseases. In the nineteenth century, Na[AuCl4] was reported to treat syphilis, whilst others used it to cure alcoholism. On a more serious note, Koch discovered in 1890 the antibacterial properties of gold cyanide. In vitro experiments with the Mycobacterium tuberculosis showed that gold cyanide has the potential as a tuberculosis therapy. Gold compounds were also investigated for the treatment of RA, when it was believed that RA was caused by bacteria, and many other health problems.
Safety Profile
Poison by intravenous route. Questionable carcinogen with experimental tumorigenic data by implantation. Can form explosive compounds with NH3, NH4OH + aqua regia, H2O2. Incompatible with mixtures containing chlorides, bromides, or iocbdes (if they can generate nascent halogens), some oxidizing materials (especially those containing halogens), alkali cyanides, thiocyanate solutions, and double cyanides. See also GOLD COMPOUNDS.
Structure and conformation
The space lattice of gold belongs to the cubic system, and its face-centered cubic lattice has a lattice constant of a=0.40705 nm.
Toxicity evaluation
The main mechanism believed to be responsible for gold salt toxicity is the formation of gold–protein complexes that elicit immune reactions. That is, gold salts may act as a hapten with subsequent antibody production against the gold–protein complex. The gold–protein–antibody complexes may in turn accumulate in the glomerular subepithelium. A second possible mechanism of gold salt toxicity is that antibodies may be formed against kidney tubular cells damaged by gold.
GOLD Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21629 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29860 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49732 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7863 | 58 |
| RongNa Biotechnology Co.,Ltd | +86-86-13583358881 +8618560316533 | Brad@rongnabiotech.com | China | 3084 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10473 | 58 |
Related articles
- The Refining methods of gold
- After initial production, gold is often subsequently refined industrially by the Wohlwill process, which is based on electroly....
- May 31,2024
- Does Gold Rust or Tarnish?
- Pure gold, being a noble precious metal, stands as the least reactive among all metals. In simple terms, pure gold does not ru....
- Mar 21,2024
- Industrial Applications of Gold
- Gold’s chemical and physical properties make it a very versatile element. Its noncorrosivenature provides protection as platin....
- Oct 8,2019
Related Qustion
- Q:Does pure gold oxidise?
- A:Yes, Gold can lose electrons under special circumstances and can therefore be oxidised. However, gold is not oxidised by oxyge....
- Mar 14,2024
View Lastest Price from GOLD manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-17 | GOLD
7440-57-5
|
US $1.10 / g | 1g | 99.0% min | 100 tons min | Shaanxi Dideu Medichem Co. Ltd | |
 |
2021-08-31 | Gold
7440-57-5
|
US $50.00 / KG | 1Kg/Bag | 99.99% | 20 tons/month | Wuhan Monad Medicine Tech Co.,LTD | |
 |
2019-07-06 | GOLD
7440-57-5
|
US $1.00 / KG | 1KG | 98% | 1kg,5kg,100kg | Career Henan Chemical Co |