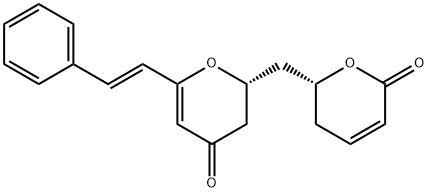
Obolactone
- CAS No.
- 712272-88-3
- Chemical Name:
- Obolactone
- Synonyms
- Obolactone;(6R)-6-[[(2R)-3,4-Dihydro-4-oxo-6-[(1E)-2-phenylethenyl]-2H-pyran-2-yl]methyl]-5,6-dihydro-2H-pyran-2-one;2H-Pyran-2-one, 6-[[(2R)-3,4-dihydro-4-oxo-6-[(1E)-2-phenylethenyl]-2H-pyran-2-yl]methyl]-5,6-dihydro-, (6R)-
- CBNumber:
- CB92725441
- Molecular Formula:
- C19H18O4
- Molecular Weight:
- 310.34
- MDL Number:
- MFCD32644959
- MOL File:
- 712272-88-3.mol
Obolactone Chemical Properties,Uses,Production
Definition
ChEBI: Obolactone is a pyranone isolated from the trunk barks of Cryptocarya obovata and has been shown to exhibit cytotoxicity against the KB cell line. It has a role as an antineoplastic agent and a plant metabolite. It is a member of 2-pyranones and a member of 4-pyranones.
Synthesis
The synthesis of (+)-obolactone (14, Scheme 3) by Brückner and Walleser
employed the same conditions from the synthesis of 17-deoxyroflamycoin to transform bis-epoxide R,R-4 to bis-homoallylic diol S,S-8. Diol 8 was then
protected using 2,2-dimethoxypropane under acidic conditions to provide
acetonide 12 in 95% yield. One of the alkene functional groups of the C2-
symmetric acetal underwent a subsequent symmetry-breaking Wacker
oxidation. Treatment of acetonide 12 with catalytic PdCl2 under an
atmosphere of oxygen using CuCl as the stoichiometric oxidant afforded a
64% yield of methyl ketone 13, with over-oxidation to the diketone also
observed (18% yield). The methyl ketone functionality of 13 was critical for
the installation of the dihydro-g-pyranone moiety in the natural product,
while the syn-orientation of the C–O bonds was achieved through Mitsunobu
inversion of the lactone stereocenter. Brückner and Walleser specifically
mention that while Krische and co-workers have reported on an impressive
single-step procedure for the catalytic enantioselective synthesis of bishomoallylic diol (S,S-4) from 1,3-propanediol, and have used
this method extensively in the synthesis of polyketide natural products,
21,22
the high cost of catalyst and ligand precluded their use on scale in this case.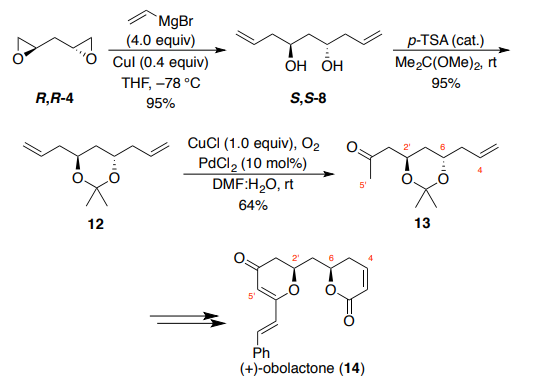
Obolactone Preparation Products And Raw materials
Raw materials
Preparation Products
Obolactone Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38630 | 58 | |
| BioBioPha Co., Ltd. | 0871-65217109 13211707573; | y.liu@mail.biobiopha.com | China | 5653 | 65 |
| Sichuan Wei Keqi Biological Technology Co., Ltd. | 028-81700200 18116577057 | 3003855609@qq.com | China | 7787 | 56 |
| Wuxi Zhongkun Biochemical Technology Co., Ltd. | 0510-85629785 18013409632 | sales@reading-chemicals.com | China | 15178 | 58 |
| EMMX Biotechnology LLC | 888-539-0666 | info@emmx.com | United States | 8447 | 60 |
| Shanghai YuanYe Biotechnology Co., Ltd. | 021-61312847; 18021002903 | 3008007409@qq.com | China | 27313 | 60 |
| Shanghai Binsui Biotechnology Co., Ltd. | 18301908279 18301908279 | 1292752157@qq.com | China | 7744 | 58 |
| Shanghai Bohu Biotechnology Co., LTD | 021-57763112 13585886131 | 3004987436@qq.com | China | 9693 | 58 |
| Wuxi Helen Biotechnology Co., Ltd., | 0510-85629785 18013409632 | sales@reading-chemicals.com | China | 14081 | 58 |
| Beijing PUXI Technology Co., Ltd | 400-8087372 13161114455 | puxi_sale@qq.com | China | 4990 | 58 |