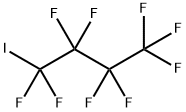
Perfluorobutyl iodide
- CAS No.
- 423-39-2
- Chemical Name:
- Perfluorobutyl iodide
- Synonyms
- NONAFLUOROBUTYL IODIDE;NONAFLUORO-1-IODOBUTANE;1,1,1,2,2,3,3,4,4-NONAFLUORO-4-IODOBUTANE;C1014NH3;uorobutyL;Perfluoroiodobutane;PERFLUOROBUTYL IODODE;PERFLUOROBUTYL IODIDE;1-Iodoperfluorobutane;1-IODONONAFLUOROBUTANE
- CBNumber:
- CB9474482
- Molecular Formula:
- C4F9I
- Molecular Weight:
- 345.93
- MDL Number:
- MFCD00001062
- MOL File:
- 423-39-2.mol
- MSDS File:
- SDS
| Melting point | -88 °C |
|---|---|
| Boiling point | 66-67 °C |
| Density | 2.01 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | None |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| form | Liquid |
| Specific Gravity | 2.010 |
| color | Clear purple |
| Water Solubility | immiscible |
| Sensitive | Light Sensitive |
| BRN | 1777546 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Stability | Stable. Incompatible with bases. |
| InChIKey | PGRFXXCKHGIFSV-UHFFFAOYSA-N |
| CAS DataBase Reference | 423-39-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | N4357HS8BY |
| NIST Chemistry Reference | 1-Iodononafluorobutane(423-39-2) |
| EPA Substance Registry System | Butane, 1,1,1,2,2,3,3,4,4-nonafluoro-4-iodo- (423-39-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H411 | |||||||||
| Precautionary statements | P273-P391-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| RIDADR | 2810 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EK5360000 | |||||||||
| F | 8 | |||||||||
| Hazard Note | Irritant/Light Sensitive | |||||||||
| TSCA | T | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29034700 | |||||||||
| NFPA 704 |
|
Perfluorobutyl iodide price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 317845 | Nonafluoro-1-iodobutane 98% | 423-39-2 | 25g | $70.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 317845 | Nonafluoro-1-iodobutane 98% | 423-39-2 | 100g | $182 | 2024-03-01 | Buy |
| TCI Chemical | N0499 | Nonafluorobutyl Iodide >98.0%(GC) | 423-39-2 | 25g | $42 | 2024-03-01 | Buy |
| TCI Chemical | N0499 | Nonafluorobutyl Iodide >98.0%(GC) | 423-39-2 | 100g | $120 | 2024-03-01 | Buy |
| Alfa Aesar | A16671 | Nonafluoro-1-iodobutane, 97% | 423-39-2 | 5g | $29.65 | 2024-03-01 | Buy |
Perfluorobutyl iodide Chemical Properties,Uses,Production
Description
Perfluorobutyl iodide (PFBI) is a promising alternative to chlorofluorocarbon solvents used in aircraft ground maintenance operations and other military and commercial operations because it cleans well, has zero ozone depletion potential, and has extremely low global warming properties. Perfluorobutyl iodides also could used as gain media for a solar-pumped laser amplifier. This compound is usually utilized as perfluoroalkyl radical precursors[1–3].
Chemical Properties
clear colourless liquid with pungent odour
Uses
Perfluorobutyl Iodide is used as an organocatalyst in some polymerization reactions.
Synthesis
Add 10 mol of triphenylphosphine chloride and 50 mol of N-methylimidazole hydrochloride to methanol and stir to dissolve. Heat to 50°C, add 65 moles of aluminum chloride, stir and dissolve, continue the reaction for 2 hours, raise the temperature to 80°C, evaporate the solvent, and filter to remove the insoluble matter to obtain quaternary ammonium salt and N-alkyl imidazole salt composite aluminum acid. Salt ionic liquid. Add 2 kg of the aluminate ionic liquid prepared in step S1 to the reaction kettle equipped with a reflux device, then add 264 g of perfluoropentanoic acid and raise the temperature to 80°C. Slowly add 2.5 mol of iodine ethanol solution dropwise within 5 hours. After the dropwise addition is completed, heat to 100°C for 8 hours. The reaction solution was allowed to stand and separated into layers to separate the aluminate ionic liquid and product layers. The product layer was purified by distillation to obtain 336g of Perfluorobutyl iodide.
References
[1] Darol Dodd, Colin Hardy, Gary Hoffman. "Perfluoro-n-butyl iodide: acute toxicity, subchronic toxicity and genotoxicity evaluations. " International Journal of Toxicology 23 4 (2004): 249–58.
[2] J. Lee, B. Tabibi, W. Weaver. "Perfluorobutyl iodides as gain media for a solar-pumped laser amplifier." Optics Communications 67 1 (1988): 435–440.
[3] Zhang, Juan et al. "Perfluorobutyl iodide-assisted direct cyanomethylation of azoles and phenols with acetonitrile?." RSC Advances 26 (2015): 20562–20565.
Perfluorobutyl iodide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Tianjin Huiren Chemtech Co., Ltd | +86-86-22-23707858 +86-86-13672008665 | huirentech@foxmail.com | China | 171 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20287 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18777 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9210 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29882 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
View Lastest Price from Perfluorobutyl iodide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-11 | Perfluorobutyl iodide
423-39-2
|
US $49.60 / KG | 1KG | 99% | 5000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-01-14 | Perfluorobutyl iodide
423-39-2
|
US $5.00-0.50 / KG | 1KG | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2023-04-29 | Perfluorobutyl iodide
423-39-2
|
US $0.00 / KG | 1KG | 99% | 10000 | Wuhan Silworld Chemical Co.,Ltd |
-

- Perfluorobutyl iodide
423-39-2
- US $49.60 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Perfluorobutyl iodide
423-39-2
- US $5.00-0.50 / KG
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Perfluorobutyl iodide
423-39-2
- US $0.00 / KG
- 99%
- Wuhan Silworld Chemical Co.,Ltd
423-39-2(Perfluorobutyl iodide)Related Search:
1of4