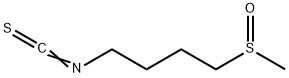
DL-Sulforaphane
- CAS No.
- 4478-93-7
- Chemical Name:
- DL-Sulforaphane
- Synonyms
- sulforaphane;SULPHORAPHANE;sulforaphan;-SuL;foraphane;sulforafan;Sulforaphen;Detoxophane;sulforphane;S-SULFORAPHANE
- CBNumber:
- CB9765544
- Molecular Formula:
- C6H11NOS2
- Molecular Weight:
- 177.29
- MDL Number:
- MFCD00198068
- MOL File:
- 4478-93-7.mol
- MSDS File:
- SDS
| Boiling point | 125-135°C |
|---|---|
| Density | 1.17±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: 40 mg/mL, soluble |
| form | liquid |
| color | slightly yellow |
| Stability | Light Sensitive |
| LogP | 0.407 (est) |
| CAS DataBase Reference | 4478-93-7(CAS DataBase Reference) |
| FDA UNII | 41684WL1GL |
| NCI Drug Dictionary | sulforaphane |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 | |||||||||
| Safety Statements | 23-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29309090 | |||||||||
| NFPA 704 |
|
DL-Sulforaphane price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S4441 | D,L-Sulforaphane ≥90% (HPLC), synthetic, liquid | 4478-93-7 | 5mg | $144 | 2024-03-01 | Buy |
| Sigma-Aldrich | 574215 | D,L-Sulforaphane | 4478-93-7 | 25mg | $280 | 2024-03-01 | Buy |
| Cayman Chemical | 10496 | Sulforaphane ≥98% | 4478-93-7 | 5mg | $41 | 2024-03-01 | Buy |
| Cayman Chemical | 10496 | Sulforaphane ≥98% | 4478-93-7 | 10mg | $78 | 2024-03-01 | Buy |
| Cayman Chemical | 10496 | Sulforaphane ≥98% | 4478-93-7 | 25mg | $183 | 2024-03-01 | Buy |
DL-Sulforaphane Chemical Properties,Uses,Production
Description
Sulforaphane is a compound within the isothiocyanate group of organosulfur compounds and mainly found in cruciferous vegetables. It is produced when the enzyme myrosinase transforms glucoraphanin,a prodrug or storage form of SFN, into SFN upon damage to the plant (such as from chewing),which allows the two compounds to mix and react. Glucoraphanin is one of a few molecules known as isothiocyanates, existing alongside Sinigrin (metabolized into allylisothiocyanate).
Chemical Properties
DL-Sulforaphane (SFN) is a slightly yellow liquid. soluble in DMSO, methanol, or water-like solvents. SFN exists in food in its food-bound form known as Glucoraphanin. It is present in a wide range of vegetables such as cabbage, cauliflower, bok choy, kale, chinese broccoli, mustard, turnip, radish, and watercress.
Uses
Potent, selective inducer of phase II detoxification enzymes with anticarcinogenic properties. Occurs naturally in broccoli. It was found to inhibit chemically induced mammary tumor formation in rats. Antitumor agent
LNCaP prostate cancer cells were treated with DL-Sulforaphane to study the effect on androgen receptor. Effects on malathion toxicity was studied in rats by treating them with DL-Sulforaphane.
Nrf2 activation of the antioxidant response element (ARE) is central to cytoprotective gene expression against oxidative and/or electrophilic stress.
Unless activated by inflammatory, environmental, or oxidative stressors, Nrf2 is sequestered in the cytoplasm by its repressor, Keap1.
Because of its protective capabilities, small molecules that activate Nrf2 signaling are being examined as potential anti-cancer or anti-inflammatory agents.
Sulforaphane is an isothiocyanate derived from cruciferous vegetables, including broccoli, that potently induces chemopreventative enzymes via Keap1-Nrf2 signaling and ARE-driven gene expression.
At 15 μM, sulforaphane inhibits class I and II HDAC activity and suppresses tumor growth by inducing cell cycle arrest and apoptosis selectively in various cancerous prostate epithelial cells without affecting normal cells.
Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells
Definition
ChEBI: Sulforaphane is an isothiocyanate having a 4-(methylsulfinyl)butyl group attached to the nitrogen. It has a role as an antineoplastic agent, a plant metabolite, an antioxidant and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is a sulfoxide and an isothiocyanate.
benefits
Sulforaphane(SFN) promotes detoxification,prevents and combats cancer,lowers cholesterol,improves diabetes, can boost the immune system, is antiviral, antibacterial, and antifungal, combats inflammation,protect skin,eyes,kidneys, and brain, and restores its cognitive function. Deep insight in several studies has reported that dietary intake of cruciferous vegetables has a direct association in the decline in the incidence of various tumors such as prostate, cervical, ovarian, and lung cancer. It improves the liver function, attenuates pain, improves hair growth,promotes bone formation, and prevents muscle damage.SFN treatment reducesDNA damage and mutation rate when cancer-causing chemicals bind DNA.Although an ideal dosage is not known, the dietary addition of 0.1-0.5mg/kg SFN to rats has been noted to be bioactive. This is an estimated human dose of 7-34mg for a 1501b person; 9-45mg for a 2001b person; 11-57mg for a 250lb person.
General Description
An isothiocyanate isolated from broccoli that acts as a potent inducer of phase II detoxifying enzymes in mouse tissues and murine hepatoma cells in culture. It has been shown to be an effective agent in prevention of chemically-induced mammary tumors in rats. It also inhibits the phase I cytochrome P450 isoenzymes 2E1 and IA2 which have been associated with the activation of carcinogens. The induction of phase II enzymes is mediated by mitogen-activated protein kinase (MAPK) pathway.
Biological Activity
Selective inducer of phase II detoxification enzymes with anticarcinogenic properties. Organosulfur compound found in cruciferous vegetables, including broccoli.
Sulforaphane is an anti-cancer, anti-microbial and anti-diabetic compound found in cruciferous vegetables. It induces the production of detoxifying enzymes such as quinone reductase and glutathione S-transferase that cause xenobiotic transformation. Sulforaphane also increases the transcription of tumor suppressor proteins and inhibits histone deacetylases. It modulates inflammatory responses by suppressing the LPS-mediated expression of iNOS, COX-2, IL-1β and TNF-α.
Anticancer Research
Sulforaphane is an isothiocyanate compound found in cruciferous vegetables like broccoli,Brussels sprouts, and cabbages. It induces phase II drug metabolism enzymes ofxenobiotic transformation and enhances the transcription of tumor suppressionproteins. It promotes cytotoxicity in p53-deleted colon cancer cells by mitochondriaandlysosome-dependent cell death. Due to the effect of sulforaphane, Bax is alsobeing increased in the presence of inhibition of JNK-induced Bcl-2 followed bymitochondrial cytochrome-C release and activation of apoptosis. Self-renewal Wnt/β-catenin signaling pathway is downregulated by sulforaphane in breast cancer stemcells. It has been reported to inhibit the activity of histone deacetylase (HDAC) andto reduce the number of polyps in Apcmin/+ mouse by inhibiting AKT and ERKsignaling and protein expression of COX-2 and cyclin-D1. Sulforaphane alsoinhibits the growth of SW620 cells by inducing apoptosis (Clarke et al. 2008). Inhuman colon cancer cells (HT-29), sulforaphane showed increased dose-dependentluciferase activity of AP-1, induced JAK activity, and inhibited NF-κB luciferaseactivation induced by LPS. It is also reported to inhibit cellular proliferation and toinduce apoptosis. In HepG2 human hepatoma cells, sulforaphane significantlyinduces the expression of the Nrf-2 protein and activation of ARE-mediatedtranscription, delays Nrf-2 degradation by inhibition of Keap1, and activates theexpression of transcription of the antioxidant HO-1 enzyme. This activation of thetranscription is partially modulated by the signaling pathway of p38 MAPK, whilep38 MAPK phosphorylates Nrf-2 and improves the binding between the proteinsNrf-2 and Keap1. In the PC-3 cells of human prostate cancer, sulforaphanesuppresses the expression regulated by NF-κB and NF-κB signaling pathway by theIκBα and IKK pathways (Wang et al. 2012).
target
Akt | ERK | TNF-α | p65 | NF-kB | IkB | COX | Nrf2 | HO-1 | Bcl-2/Bax | Caspase | AChR | IKK
References
[1] gamet-payrastre l, li p, lumeau s, et al. sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in ht29 human colon cancer cells[j]. cancer research, 2000, 60(5): 1426-1433.
[2] kansanen e, kuosmanen s m, leinonen h, et al. the keap1-nrf2 pathway: mechanisms of activation and dysregulation in cancer[j]. redox biology, 2013, 1(1): 45-49.
DL-Sulforaphane Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chengdu Greenpure Biopharma CO.,Ltd | 18283602253; +8618283602253 | jancyzheng@gcgreenpure.com | China | 953 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12840 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-18791163155 +86-13119157289 | 13119157289@163.com | China | 2973 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 377 | 58 |
| Hangzhou Lin'an tianhong biotechnology co.,ltd. | +86-0571-58605786 +8617758008775 | admin@tianhongsw.com | China | 43 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | niki@zlchemi.com | China | 771 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
Related articles
- Sulforaphane: A Potent Phytochemical with Remarkable Benefits
- Sulforaphane stands out as a highly beneficial phytochemical with diverse biological activities that offer significant health ....
- Nov 1,2024
View Lastest Price from DL-Sulforaphane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-25 | L-Sulforaphane
4478-93-7
|
US $0.00 / Kg/Bag | 2Kg/Bag | 98% up | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-22 | DL-Sulforaphane
4478-93-7
|
US $0.00 / g | 1g | 95% 98% | 100g | Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | |
 |
2024-11-21 | Sulforaphane Powder
4478-93-7
|
US $150.00 / kg | 1kg | 99% | 500kg | Hebei Zhuanglai Chemical Trading Co Ltd |
-

- L-Sulforaphane
4478-93-7
- US $0.00 / Kg/Bag
- 98% up
- Sinoway Industrial co., ltd.
-

- DL-Sulforaphane
4478-93-7
- US $0.00 / g
- 95% 98%
- Shaanxi Cuikang Pharmaceutical Technology Co., Ltd
-

- Sulforaphane Powder
4478-93-7
- US $150.00 / kg
- 99%
- Hebei Zhuanglai Chemical Trading Co Ltd