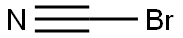Cyanogen bromide
- CAS No.
- 506-68-3
- Chemical Name:
- Cyanogen bromide
- Synonyms
- BrCN;Cyanic bromide;BROMOCYANE;carbononitridic bromide;BROMOCYANIDE;Bromocyanogen;Cyanogens bromide;tl822;(CN)Br;TL 822
- CBNumber:
- CB9853692
- Molecular Formula:
- CBrN
Lewis structure

- Molecular Weight:
- 105.92
- MDL Number:
- MFCD00011597
- MOL File:
- 506-68-3.mol
- MSDS File:
- SDS
| Melting point | 50-53 °C (lit.) |
|---|---|
| Boiling point | 61-62 °C (lit.) |
| Density | 1.443 g/mL at 25 °C |
| vapor density | 3.65 (vs air) |
| vapor pressure | 100 mm Hg ( 22.6 °C) |
| refractive index | 1.4670 (estimate) |
| Flash point | 61.4°C |
| storage temp. | 2-8°C |
| solubility | Soluble in chloroform, dichloromethane, ethanol, diethyl ether, benzene and acetonitrile. |
| form | Solution |
| color | White |
| Odor | Penetrating odor |
| Water Solubility | decomposed slowly by cold H2O [HAW93] |
| Sensitive | Moisture & Light Sensitive |
| Merck | 14,2693 |
| BRN | 1697296 |
| Exposure limits | No exposure limit is set. However, on the basis of the exposure limits of related compounds a ceiling limit of 0.5 ppm (2 mg/m3) is recommended. |
| Stability | Stable. Reacts violently with water and with mineral and organic acids. |
| CAS DataBase Reference | 506-68-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | OS382OHJ8P |
| NIST Chemistry Reference | Cyanogen bromide(506-68-3) |
| EPA Substance Registry System | Cyanogen bromide (506-68-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H314-H400 | |||||||||
| Precautionary statements | P260-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T+,N,F,C | |||||||||
| Risk Statements | 26/27/28-34-50/53-40-11-36/37-32-51/53 | |||||||||
| Safety Statements | 53-28-36/37/39-45-60-61-26-16-7/9-29-7 | |||||||||
| RIDADR | UN 3390 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GT2100000 | |||||||||
| F | 8-17-19-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28530090 | |||||||||
| Toxicity | LCLO inhal (human) 92 ppm (398 mg/m3; 10 min) LCLO inhal (mouse) 115 ppm (500 mg/m3; 10 min) |
|||||||||
| NFPA 704 |
|
Cyanogen bromide price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 261610 | Cyanogen bromide solution 5.0M in acetonitrile | 506-68-3 | 100ml | $97 | 2024-03-01 | Buy |
| Sigma-Aldrich | 341894 | Cyanogen bromide solution 3.0M in methylene chloride | 506-68-3 | 100ml | $90.5 | 2022-05-15 | Buy |
| Alfa Aesar | 032731 | Cyanogen bromide | 506-68-3 | 5g | $29.65 | 2024-03-01 | Buy |
| Alfa Aesar | 032731 | Cyanogen bromide | 506-68-3 | 25g | $65.5 | 2024-03-01 | Buy |
| Strem Chemicals | 93-1001 | Cyanogen bromide, min. 97% | 506-68-3 | 25g | $59 | 2024-03-01 | Buy |
Cyanogen bromide Chemical Properties,Uses,Production
Description
Cyanogen Bromide is a colorless or white,volatile, crystalline solid with a penetrating odor. Molecularweight=105.93; Boiling point=61-62℃; Freezing/Melting point=52℃. Hazard Identification (based onNFPA-704 M Rating System): Health 3, Flammability 0,Reactivity 3 . Soluble in water; dangerous reaction.
Chemical Properties
Cyanogen bromide is a colorless or white, volatile, crystalline solid with a penetrating odor.
Chemical Properties
white crystalline solid
Uses
Reaction with C60Ph5Cl produces a novel phenylated isoquinolino[3′,4′:1,2][60]fullerene.1
Uses
Cyanogen bromide is used in organic synthesis and as a reagent in bioanalysis.
Uses
Reagent for the synthesis of cyanamides.
Production Methods
Cyanogen bromide may be prepared by either the action of bromine on potassium cyanide or the interaction of sodium bromide, sodium cyanide, sodium chlorate, and sulfuric acid.
Synthesis Reference(s)
Journal of the American Chemical Society, 68, p. 2102, 1946 DOI: 10.1021/ja01214a501
General Description
Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. Cyanogen bromide is slightly soluble in water. Cyanogen bromide is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a flammable and poisonous gas. Contamination with many materials can cause rapid decomposition of the material. Cyanogen bromide is toxic by inhalation of its vapors or by the hydrogen cyanide from decomposition or by ingestion. Toxic oxides of nitrogen are produced in fire involving Cyanogen bromide. Cyanogen bromide is used in gold extraction, to make other chemicals, and as a fumigant.
Air & Water Reactions
Cyanogen bromide is slightly soluble in water. Cyanogen bromide is gradually decomposed by water and very rapidly by acids to give off hydrogen bromide, a poison gas.
Reactivity Profile
Cyanogen bromide is not combustible itself, but impure Cyanogen bromide decomposes rapidly and tends to explode. A violent reaction may take place on contact with large quantities of acid. Avoid physical damage, contact with acids or water, and store away from a location where water may be needed for fire control. [EPA, 1998]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).
Health Hazard
Super toxic; probable oral lethal dose in humans is less than 5 mg/kg or a taste (less than 7 drops) for a 70 kg (150 lb.) person. Vapors are highly irritant and very poisonous. Individuals with chronic diseases of the kidneys, respiratory tract, skin, or thyroid are at greater risk of developing toxic cyanide effects.
Health Hazard
Exposure to cyanogen bromide is dangerous. The chemical substance is poisonous and causes fatal injury if swallowed, inhaled, or absorbed through the skin. It is corrosive and the vapors cause severe irritation to the eyes and respiratory tract, and cause burns to any area of contact. On contact with acids, cyanogen bromide liberates poisonous gas, affecting the blood, cardiovascular system, CNS, and thyroid.
Health Hazard
Cyanogen bromide is a highly toxic substance. Its toxic effects are similar to those of HCN. However, it is not as toxic as HCN. Because it volatilizes readily at ambient temperature, inhalation is the major toxic route. The toxic symptoms in humans may be nausea, headache, and chronic pulmonary edema. Exposure to 100 ppm for 10 minutes can be fatal to humans.
LC50 inhalation (mice): 500 mg/m3/10 min
Cyanogen bromide is an irritant.
Health Hazard
The acute toxicity of cyanogen bromide is high. Toxic effects are similar to but not as severe as those of hydrogen cyanide. Toxic symptoms may include cyanosis, nausea, dizziness, headache, lung irritation, chest pain, and pulmonary edema, which may be fatal.
Cyanogen bromide may cause chronic pulmonary edema.
Fire Hazard
Cyanogen bromide is noncombustible. Impure material decomposes rapidly and can be explosive.
Fire Hazard
Cyanogen bromide is not combustible itself, but impure Cyanogen bromide decomposes rapidly and tends to explode. A violent reaction may take place on contact with large quantities of acid. Vapors are highly irritating. When material is heated to decomposition, Cyanogen bromide emits very toxic fumes of cyanide and bromide. Avoid water, acids. Avoid physical damage, contact with acids or water, and store away from a location where water may be needed for fire control.
Flammability and Explosibility
Cyanogen bromide is noncombustible. Impure material decomposes rapidly and can be explosive.
Safety Profile
A human and experimental poison by inhalation. Corrosive. When heated to decomposition it emits very toxic fumes of CNand Br-. Possibly unstable. See also other cyanogen entries; CYANIDE; and BROMIDES.
Potential Exposure
Used as an activating reagent for insoluble supports for affinity absorption. In danger are those manufacturing this compound or using it in organic synthesis or as a fumigant; in textile treatment; in gold cyaniding. It may have been used as a military poison gas.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med_x0002_ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately.Do not perform direct mouth-to-mouth resuscitation; usebag/mask apparatus. If this chemical has been inhaled,remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped.Transfer promptly to a medical facility. When this chemicalhas been swallowed, get medical attention. If victim is conscious, administer water or milk. Do not induce vomiting.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Use amyl nitrate capsules if symptoms of cyanide poisoningdevelop. All area employees should be trained regularly inemergency measures for cyanide poisoning and in CPR. Acyanide antidote kit should be kept in the immediate workarea and must be rapidly available. Kit ingredients shouldbe replaced every 1-2 years to ensure freshness. Personstrained in the use of this kit, oxygen use, and CPR must bequickly available
storage
work with BrCN should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Containers of cyanogen bromide should be kept tightly sealed and stored under nitrogen in a secondary container in a refrigerator.
Shipping
UN1889 Cyanogen bromide, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. A DOT regulated marine pollutant.
Purification Methods
All operations with this substance should be performed in a very efficient fume cupboard-it is very POISONOUS and should be handled in small amounts. Fresh commercial material is satisfactory for nearly all purposes and does not need to be purified. It is a white crystalline solid with a strong cyanide odour. If it is reddish in colour and partly liquid or paste-like, then it is too far gone to be purified, and fresh material should be sought. It can be purified by distillation using small amounts at a time, and using a short wide-bore condenser because it readily solidifies to a crystalline white solid which may clog the condenser. An appropriate gas mask should be used when transferring the molten solid from one container to another, and the operation should be done in an efficient fume cupboard. The melting point (m 49-51o) should be measured in a sealed tube. [Hartman & Dreger Org Synth Coll Vol II 150 1948.]
Incompatibilities
May be unstable unless dry and pure; impure cyanogen bromide decomposes rapidly and tends to explode. Cyanogen bromide decomposes violently on heat- ing or on contact with water, acids, or acid vapors; produc- ing highly toxic and flammable hydrogen cyanide and corrosive hydrogen bromide. Avoid physical damage, con- tact with acids or water, and store away from a location where water may be needed for fire control . Violent reaction with ammonia, amines.
Waste Disposal
May be added to strong alka- line solution of calcium hypochlorite, let stand for 24 hours and flush to sewer. May also be dissolved in flammable solvent and sprayed into an incinerator equipped with after- burner and scrubber.
Cyanogen bromide Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29811 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | 1015@dideu.com | China | 4084 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9636 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 | marketing1@neostarunited.com | China | 8838 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34553 | 58 |
Related articles
- Cyanogen bromide-Hazard and Toxicity
- Cyanogen bromide is a colorless to white crystalline solid with a penetrating odor. Cyanogen bromide is slightly soluble in wa....
- Sep 12,2019
View Lastest Price from Cyanogen bromide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-01-19 | Cyanogen bromide
506-68-3
|
US $3.00 / KG | 1KG | 98% | 1kg,5kg,50kg | Career Henan Chemical Co |
-

- Cyanogen bromide
506-68-3
- US $3.00 / KG
- 98%
- Career Henan Chemical Co