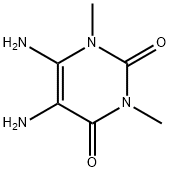
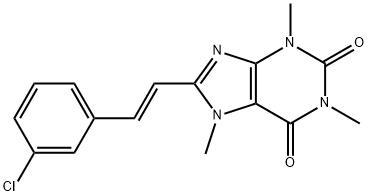
8-(3-CHLOROSTYRYL) CAFFEINE A2A ADENOSIN E RECEPTO
- CAS No.
- 147700-11-6
- Chemical Name:
- 8-(3-CHLOROSTYRYL) CAFFEINE A2A ADENOSIN E RECEPTO
- Synonyms
- (E)-8-(3-Chlorostyryl)caffeine;1,3,7-Trimethyl-8-(3-chlorostyryl)xanthine, CSC;8-(3-CHLOROSTYRYL) CAFFEINE A2A ADENOSIN E RECEPTO;8-[2-(3-Chloro-phenyl)-vinyl]-1,3,7-trimethyl-3,7-dihydro-purine-2,6-dione;(E)-8-[2-(3-Chlorophenyl)ethenyl]-3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione;1H-Purine-2,6-dione,8-[(1E)-2-(3-chlorophenyl)ethenyl]-3,7-dihydro-1,3,7-triMethyl-
- CBNumber:
- CB9972690
- Molecular Formula:
- C16H15ClN4O2
- Molecular Weight:
- 330.77
- MDL Number:
- MFCD00210188
- MOL File:
- 147700-11-6.mol
8-(3-CHLOROSTYRYL) CAFFEINE A2A ADENOSIN E RECEPTO Chemical Properties,Uses,Production
Description
8-(3-Chlorostyryl)caffeine (CSC) is an adenosine A2 receptor antagonist (Ki = 54 nM). It is selective for adenosine A2 over A1 receptors (Ki = 28 μM). CSC reverses locomotor depression induced by the adenosine A2A agonist APEC in mice (ED50 = 16 μg/kg, i.p.). It increases locomotion in mice when administered at a dose of 5 mg/kg. CSC (5 mg/kg) reverses the shortening of the rotational motor response and increases in striatal adenosine A2 receptor levels induced by L-DOPA in a rat model of Parkinson’s disease induced by 6-OHDA . It also inhibits monoamine oxidase B (MAO-B; Ki = 100 nM) and reduces MAO-B activity in brain mitochondrial preparations from wild-type and A2A-/- mice.
Uses
8-(3-Chlorostyryl)caffeine is a selective Adenosine A2A-R and MAO-B inhibitor.
Definition
ChEBI: 8-(3-chlorostyryl)caffeine is caffeine substituted at its 8-position by an (E)-3-chlorostyryl group. It has a role as an adenosine A2A receptor antagonist and an EC 1.4.3.4 (monoamine oxidase) inhibitor. It is a trimethylxanthine and a member of monochlorobenzenes. It is functionally related to a caffeine.
storage
Store at -20°C