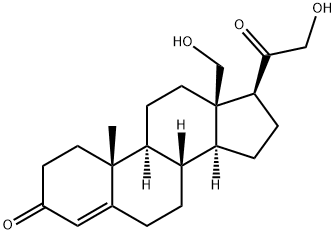
18-HYDROXY-11-DEOXYCORTICOSTERONE
- CAS No.
- 379-68-0
- Chemical Name:
- 18-HYDROXY-11-DEOXYCORTICOSTERONE
- Synonyms
- 18-HYDROXY DOC;18-HYDROXYDEOXYCORTICOSTERONE;4-PREGNEN-18,21-DIOL-3,20-DIONE;4-PREGNENE-18,21-DIOL-3,20-DIONE;18-HYDROXY-11-DEOXYCORTICOSTERONE;11-DEOXY-18-HYDROXYCORTICOSTERONE;18,21-dihydroxypregn-4-ene-3,20-dione;18,21-DIHYDROXY-4-PREGNENE-3,20-DIONE;Pregn-4-ene-3,20-dione, 18,21-dihydroxy-;18,20-EPOXY-20,21-DIHYDROXY-4-PREGNEN-3-ONE
- CBNumber:
- CB0164085
- Molecular Formula:
- C21H30O4
- Molecular Weight:
- 346.46
- MOL File:
- 379-68-0.mol
- Modify Date:
- 2023/5/25 18:00:55
18-HYDROXY-11-DEOXYCORTICOSTERONE Chemical Properties,Uses,Production
Description
18-hydroxy-11-deoxy Corticosterone (18-OH-DOC) is a mineralocorticoid secreted by the zona fasciculata of the adrenal gland. Its biosynthesis is regulated by adrenocorticotropic hormone (ACTH; ) as well as angiotensin II , which increases 18-OH-DOC production in isolated human adrenal glomerulosa cells. 18-OH-DOC can be formed via conversion of 11-deoxy corticosterone (DOC; ) in human SK-MEL188 melanoma cells. 18-OH-DOC is an intermediate in the metabolism of progesterone and can be converted to aldosterone by the capsular portion of rat adrenal glands. Continuous infusion of 18-OH-DOC (200 μg/rat per day) increases systolic blood pressure in uninephrectomized saline-drinking rats. Plasma levels of 18-OH-DOC are elevated in a db/db mouse model of type 2 diabetes.
Uses
11-Deoxy-18-hydroxycorticosterone is an analog of Corticosterone (C695700); a glucocorticoid and intermediate in the biosynthesis of Aldosterone (A514700) which is an adrenocortical steroid isolated from the adrenal cortex.
Purification Methods
Recrystallise 18-hydroxy-11-deoxycorticosterone from Et2O/Me2CO to give crystals m 200-205o. When it is recrystallised from M2CO, it has m 191-195o. It has UV with max at 240nm. The 21-O-acetoxy-18-hydroxy derivative has m 158-159o (from Et2O/*C6H6), and the 21-O-acetoxy-18,20-epoxy derivative has m 149-154o (from Et2O). [Kahnt et al. Helv Chim Acta 38 1237 1955; Pappo J Am Chem Soc 81 1010 1959.]
18-HYDROXY-11-DEOXYCORTICOSTERONE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
| ChemeGen(Shanghai) Biotechnology Co.,Ltd. | 18818260767 | China | 11289 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |
| Cayman Chemical Company | (800) 364-9897 | United States | 6618 | 81 | Inquiry |
| Shanghai Yifei Biotechnology Co. , Ltd. | 021-65675885 18964387627 | China | 8739 | 58 | Inquiry |
| BOC Sciences | USA | 0 | 65 | Inquiry | |
| Shanghai Saikerui Biotechnology Co. , Ltd. | 021-58000709 15900491054 | China | 9268 | 58 | Inquiry |
| SHANGHAI ZZBIO CO., LTD. | 13916577892 19921389125 | China | 12063 | 58 | Inquiry |
| Steraloids Inc. | 401 848-5422 | United States | 2357 | 68 | Inquiry |





