4-(Hydroxyamino)-4-oxobutanoic acid

- CAS No.
- 4743-99-1
- Chemical Name:
- 4-(Hydroxyamino)-4-oxobutanoic acid
- Synonyms
- Tofacitinib Impurity 208;N-Hydroxysuccinamidic acid;4-(Hydroxyamino)-4-oxobutanoic acid;Butanoic acid, 4-(hydroxyamino)-4-oxo-;3-(Hydroxyaminocarbonyl)propionic acid
- CBNumber:
- CB02174169
- Molecular Formula:
- C4H7NO4
- Molecular Weight:
- 133.1
- MOL File:
- 4743-99-1.mol
- Modify Date:
- 2022/8/26 12:15:11
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
4-(Hydroxyamino)-4-oxobutanoic acid Chemical Properties,Uses,Production
Preparation
To a refluxing solution of 5.0 gm (0.05 mole) of succinic anhydride in 100 ml of benzene is added a solution of 6.3 gm (0.05 mole) of O-ben- zylhydroxylamine in 20 ml of benzene. As soon as the addition has been completed, the reaction mixture is cooled, and the precipitated product is separated. Upon recrystallization from a benzene-methyl ethyl ketone mixture, 7.6 gm (68.2%) of N-benzyloxysuccinamic acid, m.p. 95-96°C isolated.
A suspension of 4.3 gm (0.0193 mole) of this intermediate and a stron- tium carbonate catalyst containing 5% palladium in methyl ethyl ketone is hydrogenated. Upon working up the reaction mixture, 1.45 gm (56.7%) of succinomonohydroxamic acid, m.p. 95-96°C is isolated.
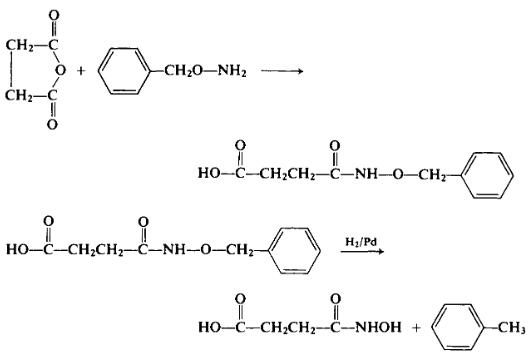
4-(Hydroxyamino)-4-oxobutanoic acid Preparation Products And Raw materials
Raw materials
Preparation Products
4-(Hydroxyamino)-4-oxobutanoic acid Suppliers
Global( 20)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Suzhou Laing Biotechnology Co.,Ltd | +86-13004516015 +86-18149392822 | China | 778 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11927 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131980 | 58 | Inquiry |
| Shanghai Sunway Pharmaceutical Technology Co.,Ltd. | 021-51816796-820 13611835272 | China | 44483 | 58 | Inquiry |
| Aikon International Limited | 025-66113011 18112977050 | China | 16028 | 58 | Inquiry |
| Bide Pharmatech Ltd. | 400-1647117 15221909166 | China | 41438 | 60 | Inquiry |
| Beijing Solarbio Science & Tecnology Co., Ltd. | 010-50973130 17801761073 | China | 50471 | 58 | Inquiry |
| Hubei Qingbei Yunyan Pharmaceutical Technology Co., Ltd | 18162595016 | China | 9887 | 58 | Inquiry |
| Cheng Du Micxy Chemical Co.,Ltd | 028-85632863 18048500443 | China | 10476 | 58 | Inquiry |
4743-99-1(4-(Hydroxyamino)-4-oxobutanoic acid)Related Search:
Tofacitinib Impurity 19
Tofacitinib impurity T
Methyl (4-Methylpiperidin-3-yl)carbaMate
4-CHLORO-7-TOSYL-7H-PYRROLO[2,3-D]PYRIMIDINE
BENZYL ETHYL ETHER
4-chloro-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine
N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine
Tofacitinib Impurity 85
N-((3R,4S)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
4-Amino-2,6-dichloropyrimidine
chevron_left
1of4
chevron_right
3-(Hydroxyaminocarbonyl)propionic acid
4-(Hydroxyamino)-4-oxobutanoic acid
N-Hydroxysuccinamidic acid
Butanoic acid, 4-(hydroxyamino)-4-oxo-
Tofacitinib Impurity 208
4743-99-1





