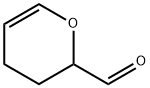
2-Formyl-3,4-dihydro-2H-pyran
- CAS No.
- 100-73-2
- Chemical Name:
- 2-Formyl-3,4-dihydro-2H-pyran
- Synonyms
- 3,4-Dihydro-2H-pyran-2-carbaldehyde;MT-A4;Acroleindimer;Pyranaldehyde;2-Propenal dimer;2,6-epoxy-5-hexena;2,6-epoxy-5-Hexenal;acrylaldehyde dimer;5-Hexenal, 2,6-epoxy-;2-formyl-2,3-dihydropyran
- CBNumber:
- CB0499031
- Molecular Formula:
- C6H8O2
- Molecular Weight:
- 112.13
- MOL File:
- 100-73-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/25 18:00:57
| Melting point | -99.9°C |
|---|---|
| Boiling point | 146 °C(lit.) |
| Density | 1.08 g/mL at 25 °C |
| refractive index | n20/D 1.466(lit.) |
| Flash point | 54 °C |
| LogP | -0.388 (est) |
| CAS DataBase Reference | 100-73-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Acrolein dimer(100-73-2) |
| EPA Substance Registry System | 2H-Pyran-2-carboxaldehyde, 3,4-dihydro- (100-73-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H318-H312-H226-H315-H335-H332 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P280-P305+P351+P338-P310-P280-P302+P352-P312-P322-P363-P501-P261-P271-P304+P340-P312 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 10-20/21/22-36/37/38-41 | |||||||||
| Safety Statements | 16-26-33-36/37/39-45 | |||||||||
| RIDADR | UN 2607 | |||||||||
| RTECS | UP6825000 | |||||||||
| HazardClass | 3.2 | |||||||||
| PackingGroup | III | |||||||||
| NFPA 704 |
|
2-Formyl-3,4-dihydro-2H-pyran Chemical Properties,Uses,Production
Chemical Properties
Colorless viscous liquid
Uses
NSC 95413 is used as a reagent in chemical processes.
General Description
A colorless or yellow liquid with a pungent disagreeable odor. Flash point 118°F. Density 1.077 g / cm3 (8.96 lb / gal). May be irritating to the eyes and mucous membranes; long-term exposure may result in adverse health effects. Vapors denser than air. Used to make other chemicals, plastics.
Air & Water Reactions
Flammable. Soluble in water.
Reactivity Profile
Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. May polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Mildly toxic by ingestion. A skin and severe eye irritant. A flammable liquid when exposed to heat, flame, or powerful oxidizing agents. To fight fire, use alcohol foam and multipurpose dry chemical. When heated to decomposition it emits acrid smoke and fumes
2-Formyl-3,4-dihydro-2H-pyran Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16209 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10259 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26165 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |





