Mupirocin
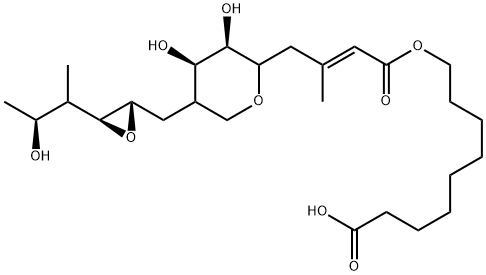
- CAS No.
- 12650-69-0
- Chemical Name:
- Mupirocin
- Synonyms
- MUPIROCIN CALCIUM;231-791-2;PSEUDOMONIC ACID;PSEUDOMONIC ACID A;bactroban;Turixi;Turixin;Mupricin;MUPIROCIN;Bactoderm
- CBNumber:
- CB0689829
- Molecular Formula:
- C26H44O9
- Molecular Weight:
- 500.62
- MOL File:
- 12650-69-0.mol
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 77-780C |
|---|---|
| alpha | D20 -19.3° (c = 1 in methanol) |
| Boiling point | 672℃ |
| Density | 1.183±0.06 g/cm3(Predicted) |
| Flash point | >110°(230°F) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: 12 mg/mL, soluble |
| form | solid |
| pka | 4.78±0.10(Predicted) |
| color | white to tan |
| optical activity | [α]/D -15.7 to -20°, c = 1 in methanol |
| Water Solubility | Soluble in DMSO or methanol. Sparingly soluble in water |
| λmax | 222nm(EtOH)(lit.) |
| Merck | 14,6302 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| InChIKey | MINDHVHHQZYEEK-HBBNESRFSA-N |
| CAS DataBase Reference | 12650-69-0(CAS DataBase Reference) |
Mupirocin price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR2806 | Mupirocin pharmaceutical secondary standard | 12650-69-0 | 100MG | ₹15490.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M7694 | Mupirocin ≥92% (HPLC), powder | 12650-69-0 | 50MG | ₹42585.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M7694 | Mupirocin ≥92% (HPLC), powder | 12650-69-0 | 100MG | ₹76651.83 | 2022-06-14 | Buy |
| TCI Chemicals (India) | M2955 | Mupirocin | 12650-69-0 | 25MG | ₹4300 | 2022-05-26 | Buy |
| TCI Chemicals (India) | M2955 | Mupirocin | 12650-69-0 | 100MG | ₹13600 | 2022-05-26 | Buy |
Mupirocin Chemical Properties,Uses,Production
Description
Mupirocin (pseudomonic acid A) is an antibiotic produced by Pseudomonas fluorescens. It is useful in the treatment of dermal infections, especially those involving S. aureus and S. epidermidis.
Chemical Properties
White Crystalline Solid
Uses
Mupirocin, is a major component of the pseudomonic acid, an antibiotic complex produced by Pseudomonas fluorescens NCIB 10586. Topical antibacterial.
Indications
Mupirocin (Bactroban, Centany) is a topical antibiotic produced by fermented
Pseudomonas fluorescens. It has a narrow spectrum of activity, mostly against
gram-positive aerobic bacteria (including Staphylococcus and methicillin-resistant
Staphylococcus) and many strains of Streptococcus. It is also active against some
gram-negative aerobic bacteria but is inactive against anaerobes, Chlamydia, and
fungi. It has proved equal in efficacy in the treatment of impetigo when compared
with oral erythromycin, with fewer adverse side effects.
Mupirocin does not interfere with wound healing. It is active only on topical
administration and is converted to an inert molecule on systemic administration.
Prolonged use of mupirocin increases the risk of evolution of resistant organisms.
Themechanism of action has not yet been fully classified, but it does differ from other
available antiinfective agents, and there is little chance of cross-resistance developing.
Also, unlike many other topical antibiotics, it rarely causes allergic sensitization.
Definition
ChEBI: An alpha,beta-unsaturated ester resulting from the formal condensation of the alcoholic hydroxy group of 9-hydroxynonanoic acid with the carboxy group of (2E)-4-[(2S)-tetrahydro-2 -pyran-2-yl]-3-methylbut-2-enoic acid in which the tetrahydropyranyl ring is substituted at positions 3 and 4 by hydroxy groups and at position 5 by a {(2S,3S)-3-[(2S,3S)-3-hydro ybutan-2-yl]oxiran-2-yl}methyl group. Originally isolated from the Gram-negative bacterium Pseudomonas fluorescens, it is used as a topical antibiotic for the treatment of Gram-positive bacterial infections.
Antimicrobial activity
It is active against staphylococci and streptococci, but also Neisseria and Haemophilus spp. Enterococcus faecalis tends to be sensitive whereas E. faecium is usually resistant. Activity against Staph. aureus is affected by inoculum such that a 10-fold increase in the inoculum causes doubling of the minimum inhibitory concentration (MIC) in vitro. Activity also decreases as pH increases above the normal skin pH of 5.5.
Acquired resistance
Before the introduction of mupirocin, resistance in Staph. aureus was uncommon, with a natural mutation frequency of 1 in 109. However, shortly after the agent was introduced, mupirocin-resistant strains began to emerge. They are of two types: low level (MIC 8–256 mg/mL) and high level (MIC >256 mg/mL).
High-level resistance, in contrast, is linked to the acquisition of a transmissible resistance gene MupA that may co-transfer with other antimicrobial resistance genes. Strains that express MupA are not clinically susceptible to mupirocin.
Several studies suggest that widespread use of prophylactic mupirocin may result in increased levels of resistance. In Canada increasing use of mupirocin across the country led to high-level mupirocin resistance, rising from 1.6% to 7% over a 9-year period.
Pharmaceutical Applications
Mupirocin is an antimicrobial substance originally derived from Pseudomonas fluorescens. It is a mixture of pseudomonic acids with more than 90% of the commercial product being pseudomonic acid A.
It has activity predominantly against Gram-positive bacteria and its main use is as a topical agent for the eradication of carriage of methicillin-resistant Staphylococcus aureus (MRSA). It is also used as a topical treatment for superficial skin infections caused by Grampositive organisms such as impetigo.
Pharmacokinetics
Following parenteral administration, mupirocin is rapidly destroyed by non-specific esterases (possibly in renal or liver tissues since it is reasonably stable in blood) to inactive monic acid and its conjugates. It is strongly protein bound. About 0.25% is absorbed from intact skin. The skin ointment, but not the cream, contains polyethylene glycol, which may be absorbed significantly when applied to open wounds or damaged skin, including burns.
Clinical Use
Mupirocin is mainly used as a nasal cream as part of the regimen to decolonize patients who have been found to carry methicillin-resistant Staph. aureus. It can also be applied to tracheostomy, gastrostomy and other sites that are frequently colonized with MRSA.
The use of mupirocin as a means of controlling outbreaks of infection due to MRSA appears to be of only marginal benefit in an endemic situation.
A Cochrane Review of nine randomized controlled trials of use of mupirocin to prevent subsequent Staph. aureus infections in nasal carriers of the organism found a statistically significant reduction in such infections at any site. A small study of local therapy to reduce the risk of peritonitis in patients on continuous ambulatory peritoneal dialysis (CAPD) found that mupirocin applied three times weekly to the dialysis catheter exit site resulted in a 92% reduction in the rate of peritonitis
Side effects
Topical applications are well tolerated. Conjunctival application is contraindicated as it may cause irritation. Minor side effects such as irritation and unpleasant or abnormal taste have been recorded for very few patients following nasal application.
Polyethylene glycol from the ointment base may, if absorbed from application to open wounds or damaged skin, cause renal toxicity.
Mupirocin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| PANCHSHEEL ORGANICS LTD | +91-9821053955 +91-9324201019 | Mumbai, India | 82 | 58 | Inquiry |
| Kawman Pharma (A Division of K P Manish Global Ingredients Pvt Ltd) | +919043474090 | Tamil Nadu, India | 16 | 58 | Inquiry |
| Horster Biotek Pvt Ltd | +91-7359657360 +91-9898127219 | Gujarat, India | 58 | 58 | Inquiry |
| Agro Cool India Ltd | +91 9999464006 | New Delhi, India | 11 | 58 | Inquiry |
| Fishfa Biogenics | +91-9978999731 +91-9978910668 | Gujarat, India | 4 | 58 | Inquiry |
| Humble Healthcare Limited | +91-9720093000 +91-8006400378 | Uttar Pradesh, India | 141 | 58 | Inquiry |
| Quad Lifesciences Pvt Ltd | +91-9810034045 +91-9810034045 | New Delhi, India | 23 | 58 | Inquiry |
| Concord Biotech Limited | +91-2714398200 +91-2714398200 | Gujarat, India | 22 | 58 | Inquiry |
| Bills Biotech Pvt Ltd | +91-8980792545 +91-8238037545 | Gujarat, India | 4 | 58 | Inquiry |
Related articles
- Perindoprilerbumine: A Comprehensive Overview
- Perindoprilerbumine is a vital medication in the treatment of hypertension, heart failure, and coronary artery disease.
- Aug 5,2024
- Mupirocin: Indications, Mechanism of action and Side Effects
- Mupirocin is a topical antibiotic analogue for the control and treatment of a wide range of skin and soft tissue infections.
- Jul 8,2024
- A naturally antibiotic:Mupirocin
- Mupirocin, formerly called pseudomonic acid A, is a naturally occurring antibiotic that was originally isolated as a fermentat....
- Mar 21,2022
12650-69-0(Mupirocin)Related Search:
1of4
chevron_right





